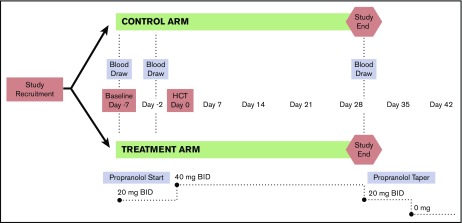
Figure 2.
Study design, intervention, and assessment schema. A phase 2 RCT was conducted to evaluate the impact of β-blocker treatment on the HSC niche among a cohort of multiple myeloma patients undergoing a first autologous HCT. Patients were treated starting 1 week prior to transplant and continuing for 4 weeks following HCT. Blood samples were collected at baseline/before drug initiation (T1), 2 days prior to HCT and prior to conditioning treatment with melphalan (T2), and at 28 days posttransplant (T3).