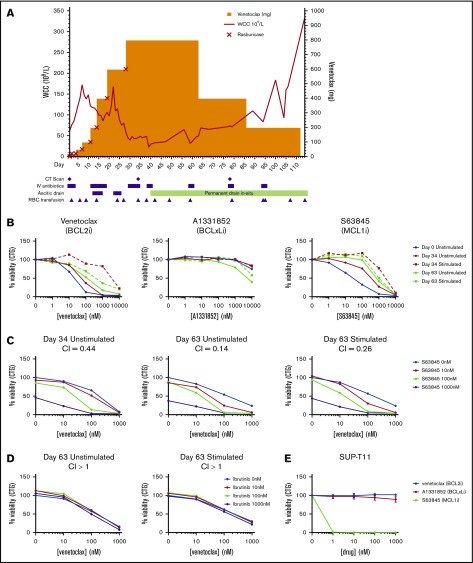
Figure 1.
Clinical course and in vitro analysis. (A) Venetoclax monotherapy dose escalation, WCC level, and clinical course. (B) T-PLL cells isolated at D0, D34, and D63 were incubated with different concentrations of venetoclax (left panel), S63845 (middle panel), or A1331852 (right panel), with and without stimulation with IL-2, IL-4, and CD40L for 24 hours before analysis of cell viability using CellTiter-Glo. (C) Unstimulated and stimulated patient cells were exposed to different concentrations of venetoclax and S63845 in combination before analysis of cell viability using CellTiter-Glo at 24 hours. (D) Unstimulated and stimulated patient cells were exposed to different concentrations of venetoclax and ibrutinib in combination before analysis of cell viability using CellTiter-Glo at 48 hours. Experiments with primary samples (B-D) were performed in triplicate, and data shown are mean values. CI values < 1 indicate synergy. (E) SUP-T11 cell line was exposed to different concentrations of venetoclax, S63845, or A1331852 before analysis of cell viability using CellTiter-Glo. Data are shown as mean ± standard deviation. n = 3.