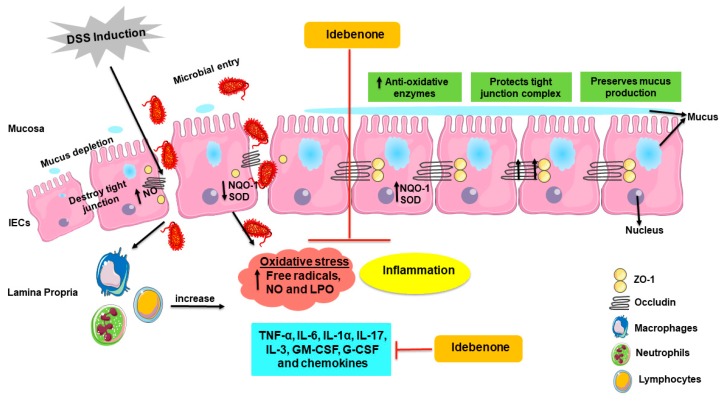
Figure 8.
Schematic illustration of the proposed mode of action of idebenone in a mouse model of DSS-induced acute colitis. Induction of DSS disrupts tight junctions (ZO-1 and occludin) and the mucus film covering epithelial cells, resulting in the increased infiltration of harmful microbes and toxins into the lamina propria. This uptake activates macrophages, neutrophils and lymphocytes, causing dissemination of pro-inflammatory cytokines (IL-6, IL-1a, TNF-a, IL-17, IL-3, GM-CSF and G-CSF), chemokines and generates oxidative and nitrosative stress (free radicals and NO). In colitis, NO is released by immune cells, as well as by IECs, which further damages tight junctions. All these factors contribute to the inflammation of the colon. Increased levels of oxidative stress via altered redox levels between oxidative molecules and anti-oxidative enzymes (NQO-1 and SOD) damages tissue and cells through oxidative damage to macromolecules, including lipids. Supplementation with idebenone maintains the barrier integrity by protecting tight junctions and the mucin layer. Idebenone also supresses the pro-inflammatory cytokines, chemokines, NO production and LPO. In addition, by increasing the levels of detoxifying enzyme NQO-1 and SOD, idebenone is thought to prevent colonic inflammation by simultaneously protecting against oxidative stress and inflammation. IECs—intestinal epithelial cells, G-CSF—granulocyte colony stimulating factor, GM-CSF—granulocyte macrophage colony stimulating factor, IL—interleukin, LPO—lipid peroxidation, NO—nitric oxide, NQO-1—NAD(P)H dehydrogenase quinone 1, SOD—superoxide dismutase, TNF-α—tumor necrosis factor alpha and ZO-1—zona occludin 1.