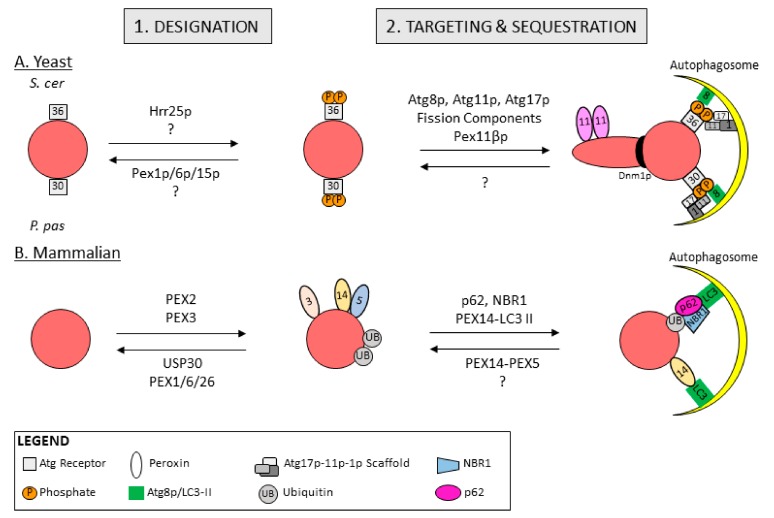
Figure 3.
Schematic of pexophagy mechanisms of designation, targeting and sequestration in yeast and mammalian systems. (A) S. cerevisiae autophagy receptor Atg36p and P. pastoris autophagy receptor Atg30p are phosphorylated by Hrr25p and an unknown kinase to designate peroxisomes for autophagy. Phosphorylated Atg36p and Atg30p interact with scaffolding proteins Atg11p, Atg17p and Atg1p that target them to autophagosomes. Interactions between phosphorylated Atg36p, Atg30p and Atg8p further sequester designated peroxisomes within autophagosomes. Pex11βp-mediated fission further aids peroxisome sequestration within the autophagosome. (B) Peroxisome membrane proteins are ubiquitinated by the E3 ubiquitin ligase, PEX2, to designate peroxisomes for pexophagy. Ubiquitinated peroxisome membrane proteins are removed from peroxisomes by the AAA-type ATPase PEX1-PEX6-PEX26 and the deubiquitinase USP30 to prevent pexophagy. Increasing expression of PEX3 on peroxisome membranes may also designate them for pexophagy. Ubiquitinated peroxisomes are targeted to autophagosomes through interactions with the autophagy receptors NBR1 and p62, which facilitate sequestration within autophagosomes through binding with LC3-II. Peroxisomes are also targeted and sequestered within autophagosomes when LC3-II out-competes PEX5 for binding to PEX14.