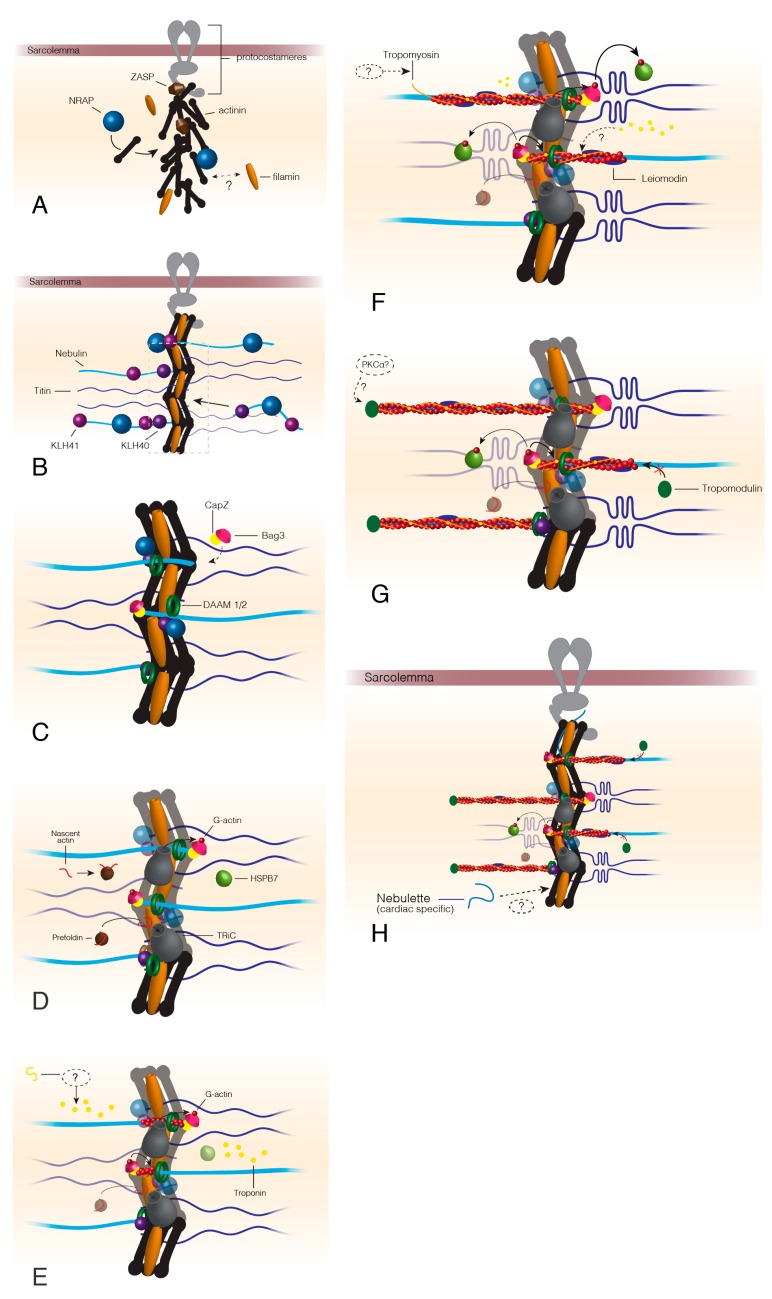
Figure 1.
Thin filament assembly beginning at the formation of the Z-disc. The formation of the Z-disc begins once ZASP is recruited to the sarcolemma via proteins of the protocostameres (A). ZASP localization draws actinin, the actinin chaperone, NRAP, and filamin C to the protocostameres (A). Actinin and filamin C organization allows for nebulin/nebulette and titin incorporation into the premature Z-disc (B). KLHL40 and KLHL41 factors stabilize nebulin as it is incorporated into the Z-disc and prevent nebulin aggregation (B). Bag3 localizes CapZ to the Z-disc at approximately the same time as DAAM1/2 appears in the Z-disc (C). Actin chaperones prefoldin and TRiC localize to the Z-disc after DAAM1/2 and Bag3. Prefoldin targets nascent actin to TRiC for folding into its final conformation. TRiC transfers folded G-actin to Bag3, which provides actin monomers for filament formation by formin proteins (D). HSPB7 arrives at the developing thin filaments around this time to regulate the speed of actin polymerization. Troponin localizes to the I-band by an unknown mechanism (E), before tropomyosin, which is also incorporated by an unknown method (F). Leiomodin is recruited to polymerizing actin thin filaments (F) and functions to both stabilize the growing filament but also prevent tropomodulin capping that stops actin polymerization (G). Once thin filaments reach their mature length, leiomodin no longer competes with tropomodulin and the thin filaments are capped by tropomodulin possibly following phosphorylation by PKCα (G,H). Dashed lines indicate unknown or hypothesized mechanisms/proteins.