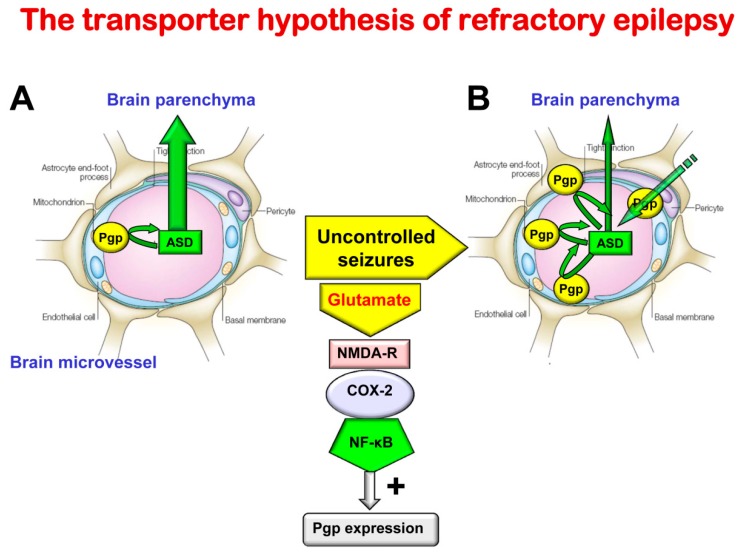
Figure 3.
Illustration of the transporter hypothesis of drug-resistant (refractory) epilepsy. About 30% of all patients with epilepsy do not respond to current ASDs and are thus drug resistant [58]. Drug resistance is associated with increased morbidity and mortality [65]. Thus, understanding the mechanisms of drug resistance is important for developing more effective therapies. The transporter hypothesis is one of several hypotheses to explain why patients do not adequately respond to treatment with ASDs. Based on the transporter hypothesis, patients with drug resistant epilepsy have an increased expression of efflux transporters at the BBB in affected brain regions, leading to reduced penetration of ASDs. Indeed, preclinical experiments have shown that sustained seizure activity leads to induction of efflux transporters such as P-glycoprotein (Pgp) at the apical (luminal) membrane of brain capillary endothelial cells that form the BBB [15,16,20]. As shown in (A), in the absence of seizures (in the nonepileptic brain) ASDs easily penetrate through the BBB by diffusion, because they are small, lipophilic, non-ionized and only relatively weak substrates of Pgp [10]. For instance, based on in vivo data with chemical knockout of Pgp in rats and genetic knockout in mice, it has been calculated that the normal, constitutive expression of Pgp at the BBB restricts brain penetration of the ASD phenytoin by about 40–50% [10]. As shown in (B), following seizures, Pgp is overexpressed in the endothelial cells, so the ASD fraction bound to Pgp in the endothelial cells increases and the drugs are transported back into the blood, thereby reducing brain levels of these drugs in affected brain regions. For instance, for phenytoin it has been shown in vivo that Pgp can affect up to about 70–80% of brain drug uptake under these conditions [10]. In addition, ASDs that are already in the brain parenchyma undergo enhanced efflux from the brain, which is mediated by Pgp. This cannot be simply counteracted by increasing the dose, because Pgp is only overexpressed in epileptogenic or focal brain tissue, so increasing the dose would lead to toxic brain levels in other regions. By inhibiting Pgp (e.g., with tariquidar), the increased ASD efflux and the associated drug resistance can be reversed in preclinical animal models of epilepsy, and, anecdotally, in patients with drug resistant seizures [10]. The mechanisms underlying the Pgp increase in response to seizures [61] are also illustrated. The increase in Pgp resulting from enhanced glutamate release in response to seizures can be inhibited by NMDA receptor antagonists or COX-2 inhibitors [10]. It is important to note that it is not likely that drug resistance is mainly due to one mechanism such as the mechanism illustrated here, but rather several resistance mechanisms have been postulated which may even occur in concert in the same patient [60,66].