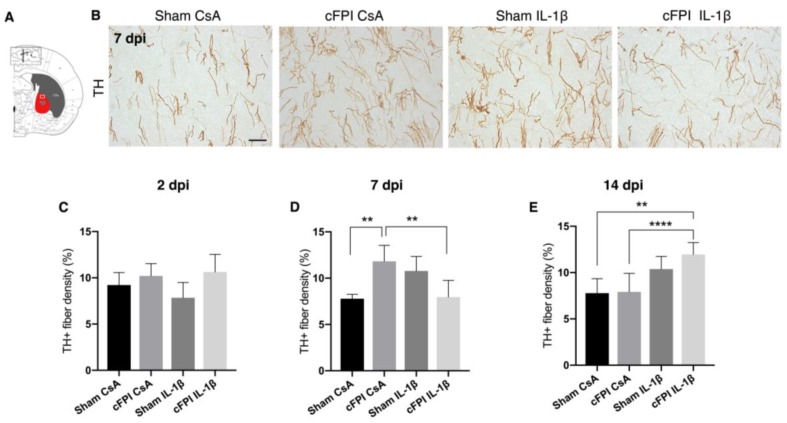
Figure 3.
Effects of diffuse traumatic brain injury and neutralizing IL-1β antibody on tyrosine hydroxylase-positive axon density in the globus pallidus. (A) Schematic representation of a coronal brain section showing location of globus pallidus (GP) and GPe (framed area in (A)). (B) Representative images showing TH+ axons in the GP at seven dpi, scale bar 20 μm. (C) Compared with control-treated, sham-injured (sham CsA) animals at two dpi, there was no change in the density of TH+ axons (sham CsA, n = 4; sham IL-1β, n = 3; cFPI CsA, n = 6; cFPI IL-1β, n = 6) (D) At seven dpi, the density of TH+ axons were increased significantly in the control-treated, brain-injured (cFPI CsA) animals in comparison to sham CsA (sham CsA, n = 4; sham IL-1β, n = 3; cFPI CsA, n = 7; cFPI IL-1β, n = 6; ** p ≤ 0.01). TH+ axon density was attenuated in brain-injured animals treated with the IL-1B neutralizing antibody. (cFPI IL-1β; ** p ≤ 0.01). (E) At 14 dpi, no difference was observed in the density of TH+ axons in the cFPI CsA animals in comparison to sham CsA animals. The density of TH+ axons was significantly increased by IL-1β neutralization in the brain-injured mice as compared to Sham CsA and cFPI CsA groups (sham CsA, n = 3; sham IL-1β, n = 3; cFPI CsA, n = 8; cFPI IL-1β, n = 11); **** p ≤ 0.001). cFPI, central fluid percussion injury; dpi, days post-injury; CsA, inactive control antibody against cyclosporin A; IL-1β, interleukin 1 beta; TH, tyrosine hydroxylase; GP, globus pallidus.