Figure. Baseline characteristics and 30-day cardiovascular and bleeding outcomes by CYP2C19 status and antiplatelet therapy.
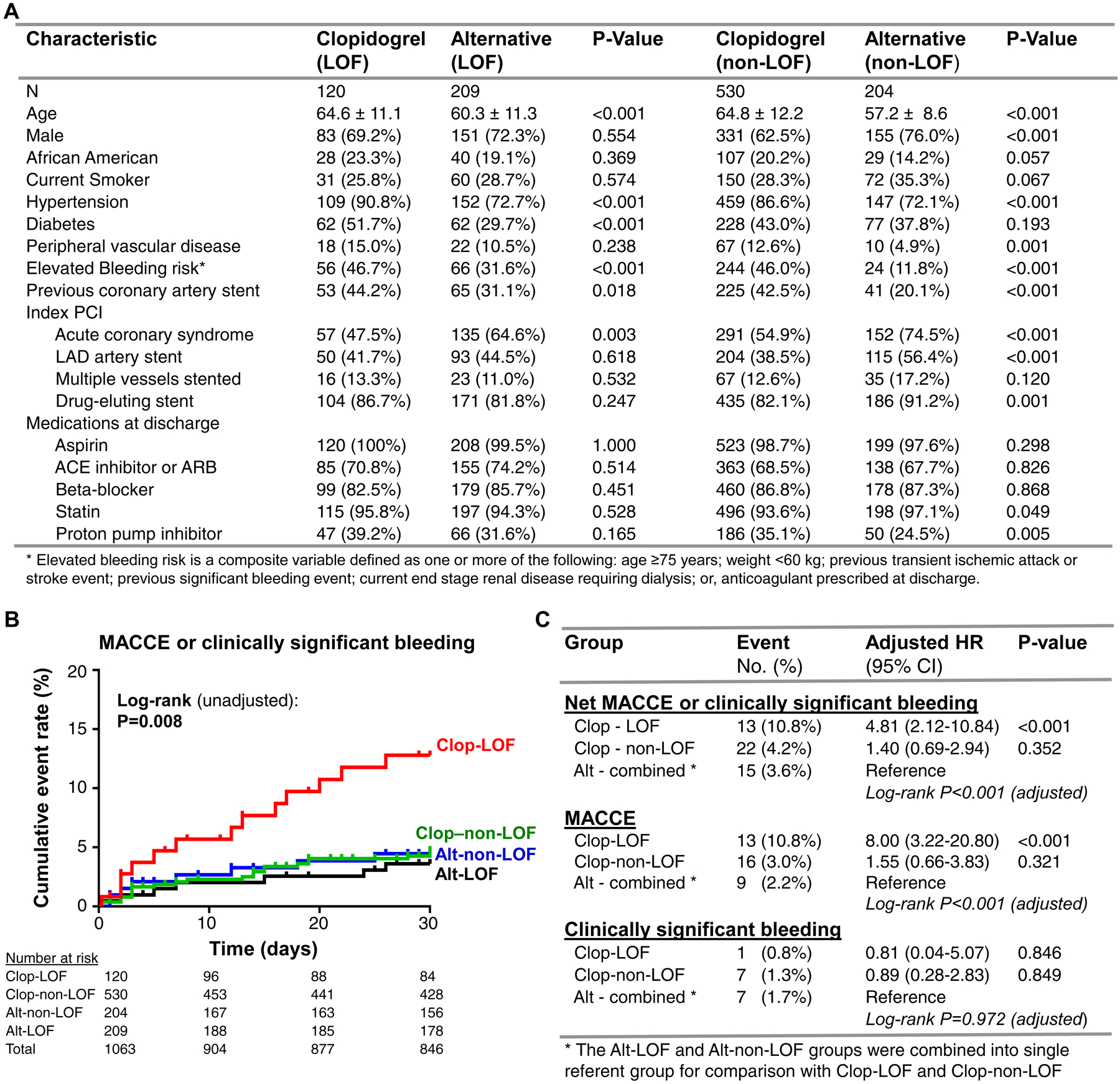
A. Baseline characteristics are presented across selected maintenance therapy (clopidogrel versus alternative [prasugrel or ticagrelor]) after stratifying by CYP2C19 loss-of-function (LOF) allele status. P-value corresponds to comparison between antiplatelet therapy groups after stratifying by CYP2C19 status using Chi-square or Fisher’s exact test. B. Kaplan-Meier curve describing 30-day cumulative event rate for the net composite of either MACCE or clinically significant bleeding across the four CYP2C19 genotype and antiplatelet therapy strata: LOF allele carriers prescribed clopidogrel (Clop-LOF), LOF allele carriers prescribed alternative therapy (Alt-LOF), patients without a LOF allele prescribed clopidogrel (Clop-non-LOF), patients without a LOF allele prescribed alternative therapy (Alt-non-LOF). The unadjusted log-rank P-value for outcomes across the four groups is provided. C. Summary of the primary (net MACCE or clinically significant bleeding) and secondary (MACCE, clinically significant bleeding) outcomes at 30 days by CYP2C19 status and antiplatelet maintenance therapy. The number (percentage) in each group that experienced the event, and the adjusted HR, 95% CI and P-value for the Cox proportional hazards regression analysis, is provided. *LOF and non-LOF allele carrier’s prescribed alternative (prasugrel or ticagrelor) therapy were combined into a single referent group for comparison. The adjusted log-rank P-value across the three CYP2C19-antiplatelet groups is provided.
