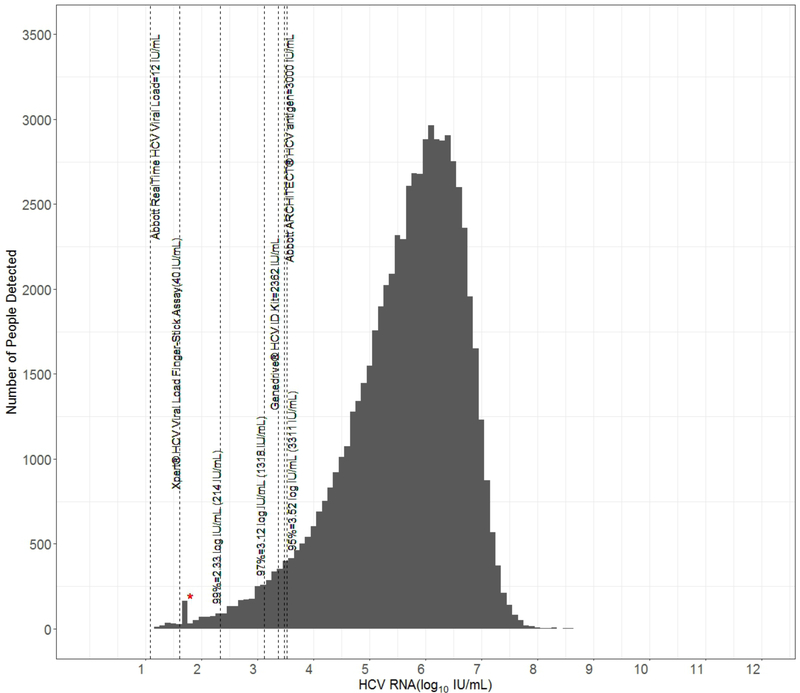
Figure 1.
Frequency distribution of HCV RNA (log10 IU/mL) among participants in a combined cross-sectional dataset from Cambodia, Canada, Cameroon, Egypt, Georgia, India, Indonesia, Malaysia, Mozambique, Pakistan, Thailand, and Vietnam with chronic hepatitis C virus (HCV). The analytic level of detection for: a) centralized nucleic acid tests (NAT) Xpert® HCV Viral Load (40 IU/mL) and Abbott RealTime HCV Viral load (12 IU/mL), b) point of care NAT Genedrive® HCV ID Kit (2362 IU/mL) and c) Abbott ARCHITECT HCV core antigen (HCVcAg) test (in IU/ml; 3000IU/ml approximately equivalent to 3 fmol/L) are marked in comparison to the limits of detection (LOD) derived for the 99th, 97th, and 95th percentiles in the dataset. *Marks the lower LOD for tests performed in India that were detected but not quantified.