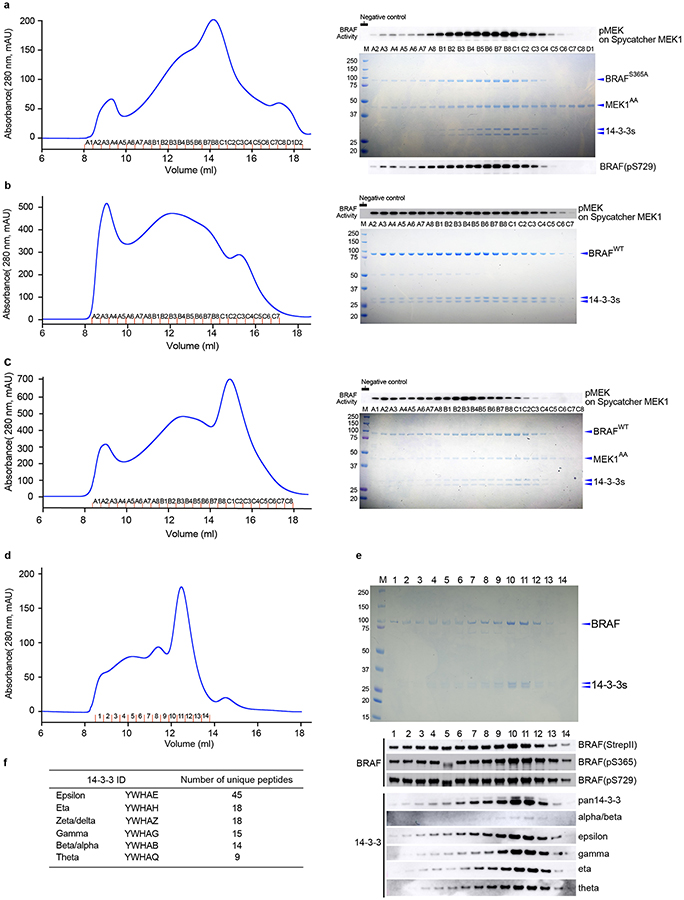
Extended Data Figure 7. Purification and characterization of wild type and S365A BRAF complexes.
a, BRAFS365A was co-expressed with MEK1AA in insect cells, purified by serial Ni-NTA agarose and StrepTrapHP affinity chromatography, and subjected to SEC on Superose 6. The SEC elution trace is shown in the left panel with a Coomassie-stained SDS-PAGE gel of elution fractions to the right. A parallel gel was blotted with an antibody against pS729 (lower right). BRAF activity in each fraction was measured in a MEK phosphorylation assay (upper right, see Methods for assay details.). b and c, Side-by-side comparison of BRAFWT complexes isolated from insect cells without (panel b) and with (panel c) co-expression of MEK1AA. Complexes were purified by serial Ni-NTA agarose and StrepTrapHP affinity chromatography and subjected to SEC on Superose 6. The SEC elution traces are shown in the left panels with Coomassie-stained SDS-PAGE gels of elution fractions to the right. BRAF activity in each fraction was measured in a MEK phosphorylation assay as described above (upper right). Note that co-expression of MEK1 markedly decreases the void peak and allows isolation of a late-eluting peak (~15 ml) with little MEK-phosphorylation activity that corresponds to the autoinhibited BRAF/MEK1/14-3-3 monomer complex (panel c, fractions B8-C3). d, BRAFWT was expressed in mammalian HEK293 cells, purified by serial Ni-NTA agarose and StrepTrapHP affinity chromatography, and subjected to SEC on Superdex 200. e, Elution fractions from the BRAFWT/14-3-3 SEC run in panel d are analyzed by SDS-PAGE and western blotting, revealing that BRAF co-purifies with endogenous human 14-3-3 proteins. Fractions were also blotted for total BRAF (anti-StrepII), pS365 and pS729. f, Mass spectrometry analysis of trypsin and Lys-C protease digests of peak fractions of the BRAF/14-3-3 complex from HEK293 cells revealed multiple peptide sequences that mapped uniquely to six of the seven human 14-3-3 isoforms. The δ and α isoforms are phosphorylation variants of ζ and β, respectively. For gel source data, see Supplementary Figure 1. SEC experiments were repeated at least 3 times (a-e), activity assays 2 (a) and 1 (b, c) times, and blotting 2 times (e) with similar results.