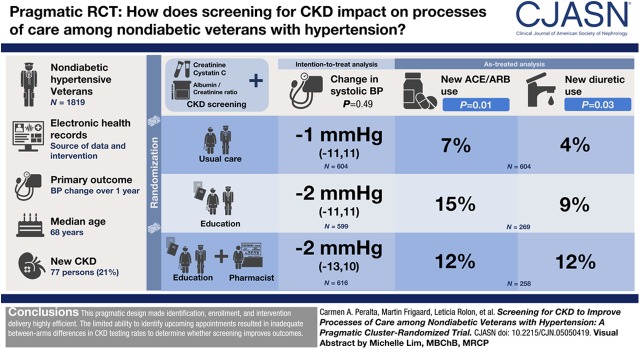
Visual Abstract
Keywords: chronic kidney disease, screening, hypertension, pragmatic trial, humans, aged, cystatin C, blood pressure, creatinine, renin-angiotensin system, diuretics, pharmacists, veterans, intention to treat analysis, process assessment (health care), blood pressure determination, electronic health records, primary health care, chronic renal insufficiency, albumins
Abstract
Background and objectives
We conducted a pilot, pragmatic, cluster-randomized trial to evaluate feasibility and preliminary effectiveness of screening for CKD using a triple-marker approach (creatinine, cystatin C, and albumin/creatinine ratio), followed by education and guidance, to improve care of hypertensive veterans in primary care. We used the electronic health record for identification, enrollment, intervention delivery, and outcome ascertainment.
Design, setting, participants, & measurements
We randomized 1819 veterans without diabetes but with hypertension (41 clusters) into three arms: (1) CKD screening followed by patient and provider education; (2) screening, education, plus pharmacist comanagement; or (3) usual care. The primary clinical outcome was BP change over 1 year. Implementation and process measures included proportion screened; CKD detection rate; and total and new use of renin-angiotensin system inhibitors, nonsteroidal anti-inflammatory drugs, and diuretics.
Results
Median age was 68 years, 55% were white, 1658 (91%) had a prior creatinine measure, but only 172 (9%) had prior urine albumin/creatinine ratio, and 83 (5%) had a prior cystatin C measure. Among those in the intervention, 527 of 1215 (43%) were identified with upcoming appointments to have CKD screening. Of these, 367 (69%) completed testing. Among those tested, 77 (21%) persons had newly diagnosed CKD. After 1 year, change in systolic BP was −1 mm Hg (interquartile range, −11 to 11) in usual care, −2 mm Hg (−11 to 11) in the screen-educate arm, and −2 mm Hg (−13 to 10) in the screen-educate plus pharmacist arm; P=0.49. There were no significant differences in secondary outcomes in intention-to-treat analyses. In as-treated analyses, higher proportions of participants in the intervention arms initiated a renin-angiotensin system inhibitor (15% and 12% versus 7% in usual care, P=0.01) or diuretic (9% and 12% versus 4%, P=0.03).
Conclusions
The pragmatic design made identification, enrollment, and intervention delivery highly efficient. The limited ability to identify appointments resulted in inadequate between-arm differences in CKD testing rates to determine whether screening improves clinical outcomes.
Introduction
Most affected persons are unaware they have CKD, despite its high burden (1,2). A strategy of early detection of CKD, followed by provider and patient education and management optimization, has the potential to reduce complications. Identification of CKD can influence decisions on hypertension treatment and targets, determine the use of renin-angiotensin system (RAS) inhibitors, inform the use of cholesterol-lowering medications (statins), and reduce the use of nephrotoxic drugs (3,4). However, there is no consensus on the value of screening for CKD in the United States. The US Preventive Services Task Force determined there was insufficient evidence to make a determination on the value of CKD screening in the general population (5). To our knowledge, no randomized trials have tested the effectiveness of CKD screening to improve care.
Early detection of CKD is also hindered by confusion regarding optimal testing strategies. An eGFR from serum creatinine (eGFRcreat) alone is insufficient to classify CKD appropriately. The international Kidney Disease Improving Global Outcomes (KDIGO) guidelines require both an estimate of eGFR and a urinary albumin/creatinine ratio (ACR) to classify risk in patients with CKD, but the guidelines do not have recommendations on screening (4). Diabetes guidelines recommend routine ACR testing. In contrast, despite the high prevalence of albuminuria among persons with hypertension, ACR quantification remains optional in the American Heart Association/American College of Cardiology hypertension guidelines (6). KDIGO guidelines suggest using eGFR estimated from serum cystatin C as a confirmatory measure when eGFRcreat is between 45 and <60 ml/min per 1.73 m2 with no evidence of kidney damage (i.e., normal ACR), and in situations where eGFRcreat may be unreliable. Our prior work has shown that an approach that includes serum creatinine and cystatin C and urinary ACR (a “triple-marker” approach) can improve detection and risk stratification compared with creatinine alone (7). The value of incorporating the triple-marker testing approach for CKD screening is unknown.
The widespread use of electronic health records has garnered great excitement about evaluating interventions that capitalize on technology to improve clinical care. In nephrology, we have limited experience with pragmatic randomized trials that have tested the potential of the electronic health record to improve management of persons at early stages of CKD (8–13). Even fewer report lessons learned in the process of implementation (14). Herein, we report on a three-arm, pilot, pragmatic, cluster-randomized trial to evaluate the feasibility and preliminary effectiveness of triple-marker screening for CKD, followed by two incremental strategies of education and guidance on management optimization, compared with usual care, to improve BP management among veterans with hypertension who are not diabetic in primary care.
Materials and Methods
Study Design
In brief, this was a pilot, pragmatic, three-arm, cluster-randomized trial in which veterans with hypertension who did not have known CKD or diabetes were randomized to one of two additive intervention arms or usual care (ClinicalTrials.gov; #NCT02059408). The study team included expertise in nephrology, primary care, health services research, implementation, informatics, data science, and biostatistics. The intervention arms began with triple-marker screening for CKD followed by provider and patient education (“screen educate”); one intervention arm additionally included an option for pharmacist comanagement (“screen educate plus pharmacist”). The setting was primary care practices at the San Francisco Veterans Affairs (VA) Medical Center. The unit of randomization was the provider team.
Patient identification and enrollment, delivery of the intervention, and ascertainment of outcomes were all conducted using the electronic health record. We designed the study protocol to function within the Patient Aligned Care Team model. The rationale and detailed methods for this trial have been previously published (15).
Inclusion and Exclusion Criteria
Providers were eligible if they had a primary care panel at the San Francisco VA. Using data from the VA Corporate Data Warehouse, we identified patients aged 18–80 years, who had documented hypertension in the past 5 years, and had a primary care visit with an eligible primary care provider in the past 18 months along with no diagnosis of CKD. We excluded those with diabetes because guidelines already recommend annual screening for CKD. Hypertension, diabetes, and CKD status were determined based on clinical documentation in the record, and using validated International Classification of Diseases, Ninth Revision, Clinical Modification codes, as previously published (15). We also excluded patients with heart failure and documented reduced ejection fraction or those for whom the primary provider deemed that screening for CKD and trial participation would not be appropriate (severe mental illness, cognitive impairment, or short life expectancy). Attending supervising providers signed informed consent. Patients received a letter allowing them the ability to opt out. This study was approved by the University of California, San Francisco Institutional Review Board and the Veterans Administration Research and Development Committee.
Randomization
Patients were randomized in clusters based on their assigned primary care team, using the SAS procedure SURVEYSELECT in a computer-generated randomization scheme stratified by team size. The final randomization plan identified clusters using unique coded alphanumeric identifiers. Due to the nature of the pragmatic interventions, providers and patients could not be blinded after randomization. The data analyst was blinded to team assignment until study completion.
Study Procedures
The study was conducted for 14 months after randomization, from February 19, 2016 to April 18, 2017. Before randomization, study nephrologists conducted a presentation on the topic of triple-marker screening for CKD, which was open to all providers. Patients who were randomized to usual care were not systematically screened for CKD.
In the screen-educate arm, study staff reviewed appointment data in the data warehouse approximately every 10 days to ascertain those with scheduled upcoming outpatient appointments and then ordered serum creatinine, cystatin C, and ACR tests. Once tests were completed, study nephrologists sent an electronic note to the primary care provider to cosign. The note included a summary of the results and corresponding guidance, depending on CKD status. Among patients with newly identified CKD (defined using the combined eGFRcreat and cystatin C equation [eGFRcreat-cys] <60 ml/min per 1.73 m2 or ACR ≥30 mg/g), the study-generated electronic note included guidance on BP targets (<140/90 mm Hg), recommendations to use RAS inhibitors in persons with albuminuria and the use of diuretics, recommendations on counseling about avoiding nonsteroidal anti-inflammatory drugs (NSAIDs), and links to international clinical practice guidelines. In addition, an automated letter was generated with the triple-marker results and education which was then mailed to the veteran.
In the screen-educate plus pharmacist intervention, the electronic letter to the primary care provider additionally included an opt-in option to refer persons with newly detected CKD to a clinical pharmacist for medication review, medication adjustment, and CKD counseling.
Data Elements
Baseline Measures.
All study data were ascertained from the VA Corporate Data Warehouse. Patient demographics included sex, age, race/ethnicity, and marital status at the time of randomization (February 18, 2016). We previously described the chart review and data-validation process (15). Baseline medication use of antihypertensives, lipid-lowering drugs, aspirin, and NSAIDs, was based on outpatient prescription fills from a VA pharmacy within 6 months before randomization. Baseline laboratory measures were based on the most recent outpatient test result within 2 years before randomization. Baseline systolic and diastolic BPs were based on the most recent BP result from a primary care clinic within 6 months before randomization.
Triple-Marker CKD Screen.
Results of laboratory screening for CKD using the triple-marker test panel were noted in the study principal investigator’s (C.A.P.) electronic inbox. Results were processed by a study nephrologist (L.R.) to determine CKD status.
Implementation Process Measures
We adapted the RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) framework (16). We previously published preliminary measures of implementation and characteristics of enrolled versus excluded or opted out (15). Herein we report on the proportion with screening tests ordered, defined as the proportion of persons randomized to the intervention arms for whom an upcoming appointment was identified and triple-marker screening was ordered. Prespecified implementation measures included proportion screened, defined as the proportion of persons randomized to the intervention arms who were successfully screened for CKD with the triple-marker tests, and CKD detection rate (yield), which was defined as number of cases of newly identified CKD (defined as eGFRcreat-cys <60 ml/min per 17.3 m2 or ACR ≥30 mg/g) among those with screening ordered and testing completed. We constructed additional exploratory implementation process outcomes, including CKD detection by individual measures among those tested: eGFRcreat <60 ml/min per 17.3 m2, eGFR by cystatin C <60 ml/min per 17.3 m2, and ACR ≥30 mg/g.
Clinical and Clinical-Process Outcomes
The primary clinical outcome was BP change, defined as the change in systolic and diastolic BP (separately) from baseline to study end. BP control (<140/90 mm Hg) at study end was a secondary clinical outcome. BP was measured by clinical personnel using automated cuffs as per routine.
The secondary process of care outcomes for effectiveness included proportion on RAS inhibitors (prespecified secondary outcome) and NSAIDs, defined as the proportion of patients in each arm with a corresponding outpatient medication fill during the study period. The proportion using diuretics was an exploratory outcome. We calculated overall use and new use, defined as use of each class of medications (RAS inhibitors, diuretics, and NSAIDs) during the study period among persons not using the class of medications at baseline. Other prespecified secondary outcomes included CKD recognition by primary care provider, defined as an outpatient diagnosis of CKD documented using International Classification of Diseases, Ninth Revision, Clinical Modification codes during the study period. We obtained information on testing charges from the laboratory (secondary outcome).
Finally, we assessed provider burden and increased CKD knowledge to assess adoption and potential sustainability of the intervention. Attending primary care providers and pharmacists who participated in the study and were still working at the clinic were e-mailed an anonymous web-based survey to assess their burden and increased knowledge of care for CKD due to the study (see Supplemental Appendix).
Analyses
We compared baseline patient characteristics by study arm using chi-squared tests or Kruskal–Wallis tests as appropriate. We first described proportions of patients in the intervention arm for whom triple-marker CKD screening tests were ordered, and the rate of test completion among patients in the intervention arm and among those with screens ordered. We described the rate of CKD detection from the triple-marker screen among patients with screens ordered and among those tested overall and by each kidney marker. Among patients in the intervention with newly detected CKD, we described the proportion for whom the primary care providers documented a diagnosis of CKD and the proportion in the screen-educate plus pharmacist arm who had a documented pharmacist visit.
We compared BP levels and the proportions of patients with total use and new use of RAS inhibitors, NSAIDs, and diuretics across the three study arms. Comparisons are presented for all participants randomized (“intention-to-treat” analyses) and among those for whom tests were ordered (“as-treated” analyses). Differences in BP levels were assessed using Kruskal–Wallis tests in the intention-to-treat analyses and using median regression in the weighted per-protocol analyses. Differences in medication use were assessed using Pearson chi-squared tests. We powered this study for changes in BP, using Stata version 11.2, assuming a two-tailed α level of 0.05 and intraclass correlation coefficient of 0.025.
The as-treated analysis was restricted to patients in the intervention for whom we identified an upcoming appointment and placed an order. Since this subset of participants showed differences in some characteristics compared with those who had missing follow-up appointments, we applied inverse probability weighting so that this subset of patients in the intervention would be representative of the patient characteristics in the overall population of all patients in the intervention (17).
We also compared primary care provider recognition of CKD among patients in the intervention with newly detected CKD versus patients receiving usual care and, in additional exploratory analyses, compared new use of RAS inhibitors, diuretics, and NSAIDs among patients in the intervention with and without newly detected CKD versus patients receiving usual care using Pearson chi-squared tests.
Finally, we compared the proportion of respondents to our provider survey who indicated that the study (1) increased their practice burden, (2) improved their knowledge of CKD management, and (3) prompted changes to patients’ care plans using Pearson chi-squared tests.
Results
Enrollment, Allocation, and Baseline Characteristics
We randomized 1819 patients, within 41 clinical teams. As previously published (15), among the 2293 persons identified through the electronic health records, 114 were excluded due to ineligible primary care provider, 138 were excluded by their primary care provider, 27 were excluded due to heart failure, two had died, and 193 opted out.
The median age was 68 years (interquartile range [IQR], 61–72 years), 55% were white, and 8 (0.4%) were female. Within the 2 years before randomization, 1658 (91%) had creatinine measured, but only 172 (9%) had ACR and 83 (5%) had cystatin C measured. Median eGFRcreat at baseline was 84 (IQR, 72–94) ml/min per 1.73 m2, and 8% (n=146) had eGFRcreat <60 ml/min per 1.73 m2. A total of 810 (45%) had prior urinalysis by dipstick. There were few differences in patient characteristics across study arms (Table 1). We previously published differences between the patients that were included and excluded in the study (15).
Table 1.
Baseline characteristics of participants in a pilot, pragmatic, cluster-randomized trial of veterans with hypertension
| Characteristics | Total (N=1819) | Usual Care (n=604) | Screen Educate (n=599) | Screen Educate and Pharmacist (n=616) |
|---|---|---|---|---|
| Demographics | ||||
| Male, n (%) | 1811 (100%) | 603 (100%) | 598 (100%) | 610 (99%) |
| Age, median (IQR) | 68 (61–72) | 68 (62–72) | 68 (61–72) | 68 (61–72) |
| Race/ethnicity, n (%) | ||||
| White | 1009 (55%) | 344 (57%) | 329 (55%) | 336 (55%) |
| Black | 298 (16%) | 87 (14%) | 107 (18%) | 104 (17%) |
| Asian/Pacific Islander | 150 (8%) | 52 (9%) | 48 (8%) | 50 (8%) |
| American Indian | 11 (1%) | 2 (0%) | 5 (1%) | 4 (1%) |
| Hispanic | 92 (5%) | 31 (5%) | 28 (5%) | 33 (5%) |
| Missing | 259 (14%) | 88 (15%) | 82 (14%) | 89 (14%) |
| Outpatient diagnoses, n (%) | ||||
| Ischemic heart disease | 341 (19%) | 121 (20%) | 106 (18%) | 114 (19%) |
| Congestive heart failure | 112 (6%) | 34 (6%) | 35 (6%) | 43 (7%) |
| Cerebrovascular disease | 200 (11%) | 75 (12%) | 63 (11%) | 62 (10%) |
| Hyperlipidemia | 1187 (65%) | 407 (67%) | 400 (67%) | 380 (62%) |
| Any malignancy | 344 (19%) | 109 (18%) | 126 (21%) | 109 (18%) |
| COPD and bronchiectasis | 375 (21%) | 125 (21%) | 104 (17%) | 146 (24%) |
| Tobacco use disorder | 513 (28%) | 168 (28%) | 176 (29%) | 169 (27%) |
| Drug use disorders | 301 (17%) | 91 (15%) | 94 (16%) | 116 (19%) |
| Alcohol use disorders | 503 (28%) | 168 (28%) | 164 (27%) | 171 (28%) |
| Mental health disorders | 823 (45%) | 281 (47%) | 270 (45%) | 272 (44%) |
| Outpatient medication fill, n (%) | ||||
| Any hypertension medications | 1277 (70%) | 429 (71%) | 412 (69%) | 436 (71%) |
| Diuretic | 435 (24%) | 150 (25%) | 150 (25%) | 135 (22%) |
| β-Blockers | 493 (27%) | 176 (29%) | 148 (25%) | 169 (27%) |
| RAS inhibitors | 629 (35%) | 213 (35%) | 201 (34%) | 215 (35%) |
| Calcium channel blocker | 506 (28%) | 153 (25%) | 174 (29%) | 179 (29%) |
| Aspirin | 226 (12%) | 72 (12%) | 79 (13%) | 75 (12%) |
| Statins | 788 (43%) | 266 (44%) | 269 (45%) | 253 (41%) |
| NSAID | 319 (18%) | 109 (18%) | 108 (18%) | 102 (17%) |
| Most recent outpatient kidney lab result | ||||
| ACR (mg/g), median (IQR) | 6.0 (3.0, 17.5) | 7.0 (3.0, 17.0) | 5.0 (3.0, 21.0) | 7.0 (4.0, 20.0) |
| Creatinine (serum, mg/dl), median (IQR) | 1.0 (0.8, 1.1) | 0.9 (0.8, 1.1) | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.1) |
| eGFRcreat (serum, ml/min per 1.73 m2), median (IQR) | 84 (72, 94) | 85 (72, 94) | 84 (72, 94) | 84 (71, 94) |
| Cystatin C (serum, mg/L), median (IQR) | 1.0 (0.9, 1.2) | 1.1 (1.0, 1.3) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) |
| Most recent BP measurement in primary care clinic | ||||
| Systolic BP (mm Hg), median (IQR) | 136 (126, 147) | 136 (126, 147) | 136 (125, 146) | 136 (126, 146) |
| Diastolic BP (mm Hg), median (IQR) | 79 (74, 85) | 79 (74, 86) | 79 (73, 85) | 79 (74, 85) |
| No. primary care visit dates with BP, median (IQR) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
Numbers do not add to total and percentages do not add to 100% due to missing values. IQR, interquartile range; COPD, chronic obstructive pulmonary disease; RAS, renin-angiotensin system; NSAID, nonsteroidal anti-inflammatory drug; ACR, albumin/creatinine ratio; eGFRcreat, eGFR by creatinine.
Reach and Adoption of Interventions (Implementation)
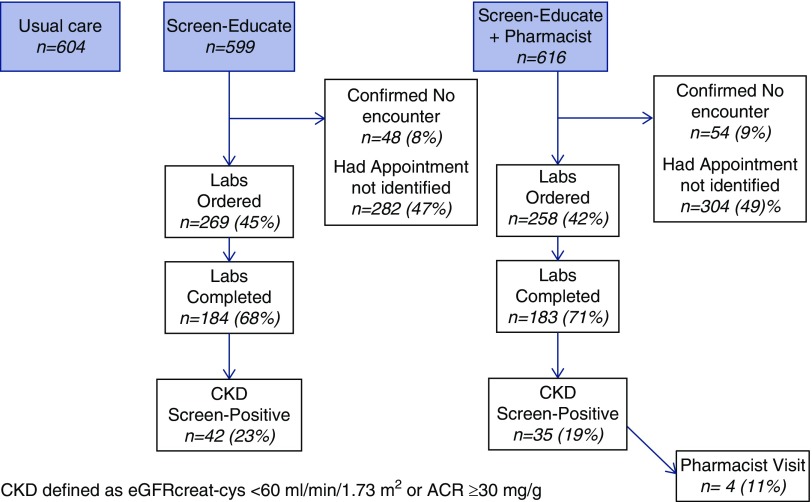
Randomization occurred on February 18, 2016 and orders for triple-marker screening were placed from February 22, 2016 to March 27, 2017. Clinical outcomes were ascertained for 14 months after randomization (February 19, 2016 to April 18, 2017). Among persons randomized to intervention arms (n=1215), we identified 527 (43%) as having an upcoming outpatient appointment and thus had an order for triple-marker testing placed using the data warehouse queries. The median time from the date the order was placed to the study end was 284 (IQR, 195–365) days. Since we failed to identify upcoming appointments for 57% of patients receiving the intervention during the study period, we requeried the appointment data to understand the process further, approximately 6 months after the study was completed. Among those for whom an appointment was not identified by initial queries of the appointment data, only 14% (96 of 688) were confirmed as having no outpatient appointment during the study period. Nearly half of all outpatient appointments were scheduled <10 days before the appointment, including >20% on the same day. Moreover, appointment data appeared to enter the data warehouse retrospectively in many cases.
Among the 1215 patients receiving the intervention, 367 (30%) were screened for CKD with the triple-marker tests. The completion rate was high once orders were placed, with 70% of those for whom the screen was ordered having a test (Figure 1). The Medicare reimbursement rate for the triple-marker set was $35.82, but costs for the VA are much lower.
Figure 1.
Study flow uptake of the intervention.
A total of 77 (21%) persons tested with the triple-marker screen were newly diagnosed with CKD. The yield was 15% among all persons with screen tests ordered (Figure 1). The uptake of the pharmacist-comanagement option was low, with a total of ten pharmacy consult orders placed, six in the screen-educate arm and four in the screen-educate plus pharmacist arm. Among the 35 persons in the screen-educate plus pharmacist arm who had newly detected CKD, only four persons (11%) had a pharmacist visit documented in the record. Of the 70 participating providers, two attendings left the VA before testing started and 14 residents graduated and their patients were reassigned as per usual practice.
Table 2 shows the distribution of the abnormal triple-marker results by marker. The most frequent abnormality that resulted in a new CKD diagnosis was the finding of albuminuria. Among 41 persons with eGFRcreat <60 ml/min per 1.73 m2, 41% did not have CKD confirmed by cystatin C or albuminuria.
Table 2.
New diagnoses of CKD according to diagnostic test
| Screens | Screening Results, n (%) (n=367a) |
|---|---|
| Abnormal screens by measure | |
| CKD (by combined equation or ACR) | 77 (21%) |
| eGFRcreat-cys <60 ml/min per 1.73 m2 | 34 (9%) |
| eGFRcreat <60 ml/min per 1.73 m2 | 41 (11%) |
| eGFRcys <60 ml/min per 1.73 m2 | 41 (11%) |
| ACR ≥30 mg/g | 54 (15%) |
| Any measure | 99 (27%) |
| Abnormal screens only by single measure | |
| eGFRcreat-cys <60 ml/min per 1.73 m2 only | 21 (6%) |
| eGFRcreat <60 ml/min per 1.73 m2 only | 17 (5%) |
| eGFRcys <60 ml/min per 1.73 m2 only | 13 (4%) |
| ACR ≥30 mg/g only | 39 (11%) |
ACR, albumin/creatinine ratio; eGFRcreat-cys, eGFR by creatinine and cystatin C; eGFRcreat, eGFR by creatinine; eGFRcys, eGFR by cystatin C.
Note that of the 367 patients in the intervention who completed CKD screening, two did not have a valid test result for creatinine, one did not have a valid test result for cystatin C, and 26 did not have a valid test result for creatinine. Therefore the “only” measures of CKD testing results are calculated among the 339 patients with valid results on all three markers.
Clinical and Process Outcomes
Primary Outcome.
Overall, 1580 participants had follow-up BPs, and the mean number of visits with BP measured was 3.08 (SD 2.29). There were no statistically significant differences between study arms in BP change in the intention-to-treat analyses (Table 3). The median (IQR) systolic BP at study end was 135 (126–146) mm Hg for usual care, 135 (126–145) mm Hg for screen-educate, and 135 (124–145) mm Hg for screen-educate plus pharmacy arms. Among persons with uncontrolled BP at baseline (>140/90 mm Hg), overall median (IQR) systolic BP change was −9.5 (−20.4 to 0.0) mm Hg. Among these 428 participants, there were no differences in systolic BP change by study arm: −10 (−20 to 0.5) mm Hg in the usual-care, −9.1 (−20 to −1.5) mm Hg in the screen-educate, and −9.5 (−20 to 0) mm Hg in the screen-educate plus pharmacist arm (P=0.86).
Table 3.
Changes in BP: primary and secondary study outcomes
| Outcome | Usual Care | Screen Educate | Screen Educate and Pharmacist | P Value |
|---|---|---|---|---|
| Intention-to-treat analyses | ||||
| Total patients (n) | 604 | 599 | 616 | |
| Primary clinical outcome, median (IQR) or N (%) | ||||
| Change in SBP from baseline (n=1118) | −1 (−11 to 11) | −2 (−11 to 11) | −2 (−13 to 10) | 0.49 |
| Change in DBP from baseline (n=1118) | −2 (−7 to 4) | −2 (−7 to 4) | −1 (−7 to 5) | 0.72 |
| Secondary clinical outcome | ||||
| Controlled BP at study end (n=1580) | 323 (62%) | 323 (61%) | 340 (64%) | 0.56 |
| As-treated analysesa | ||||
| Total patients (n) | 604 | 269 | 258 | |
| Primary clinical outcome, median (IQR) | ||||
| Change in SBP from baseline (n=749) | −1 (−11 to 11) | −3 (−11 to 9) | −2 (−12 to 10) | 0.51 |
| Change in DBP from baseline (n=749) | −2 (−7 to 4) | −3 (−9 to 4) | −1 (−7 to 5) | 0.07 |
| Secondary clinical outcome (inverse probability weighted %) | ||||
| Controlled BP at study enda | 62%a | 60%a | 61%a | 0.79 |
IQR, interquartile range; SBP, systolic BP; DBP, diastolic BP.
Per-protocol analyses were conducted in subset of patients in the intervention with CKD screening ordered; stabilized inverse probability weighting was applied to weight this subset back to the population of all patients in the intervention.
In the as-treated analyses, we first applied inverse probability weighting and showed that we achieved good relative balance of characteristics of usual care compared with patients in the intervention for whom tests were ordered (Supplemental Table 1). We found no significant differences in BP change between study arms (Table 3).
Secondary and Exploratory Outcomes.
There were no significant differences in BP control in the intention-to-treat or as-treated analyses (Table 3).
When we evaluated differences in drug use, we found no differences in overall medication use or new medication use in the intention-to-treat analyses (Table 4). In as-treated analyses, which included only participants who had a triple-marker screen ordered, we found larger proportions of patients with new use of RAS inhibitors and diuretics in the intervention arms compared with the usual-care arm (Table 4).
Table 4.
Secondary and Exploratory Process Outcomes
| Outcome | Usual Care | Screen Educate | Screen Educate and Pharmacist | P Value |
|---|---|---|---|---|
| Intention-to-treat analyses | ||||
| Total patients (n) | 604 | 599 | 616 | |
| Overall use at end of study, N (%) | ||||
| RAS inhibitors | 222 (37%) | 221 (37%) | 242 (39%) | 0.59 |
| NSAID | 146 (24%) | 150 (25%) | 148 (24%) | 0.91 |
| Diuretica | 158 (26%) | 167 (28%) | 158 (26%) | 0.66 |
| New use by end of study, N (%)b | ||||
| RAS inhibitors | 26 (7%) | 38 (10%) | 39 (10%) | 0.23 |
| NSAID | 60 (16%) | 56 (16%) | 55 (14%) | 0.82 |
| Diuretica | 20 (4%) | 31 (7%) | 37 (8%) | 0.10 |
| As-treated analysesc | ||||
| Total patients (n) | 604 | 269 | 258 | |
| Overall use at end of study (inverse probability weighted %) | ||||
| RAS inhibitors | 37% | 39% | 38% | 0.81 |
| NSAID | 24% | 31% | 26% | 0.15 |
| Diuretica | 26% | 31% | 29% | 0.33 |
| New use by end of study (inverse probability weighted %)b | ||||
| RAS inhibitors | 7% | 15% | 12% | 0.01 |
| NSAID | 16% | 15% | 15% | 0.91 |
| Diuretica | 4% | 9% | 12% | 0.003 |
RAS, renin-angiotensin system; NSAID, nonsteroidal anti-inflammatory drug.
Exploratory process outcome.
New medication use among patients not on that medication at baseline.
Per-protocol analyses were conducted in subset of patients in the intervention with CKD screening ordered; stabilized inverse probability weighting was applied to weight this subset back to the population of all patients in the intervention.
In exploratory analyses comparing patients in the intervention who completed CKD testing to patients receiving usual care, stratified by CKD status, the increased rates of new use of RAS inhibitors was limited to patients in the intervention with newly detected CKD, whereas the increased rates of new diuretic use was limited to patients receiving the intervention who tested negative for CKD (Supplemental Table 2).
In the screen-educate arm, 5% of patients who tested positive for CKD had new documentation of CKD in the problem list at study end, compared with 17% in the screen-educate plus pharmacist arm, and 2% in usual care (P<0.001).
Finally, among attending providers who remained at the clinic after study completion, 61% (n=20) responded to an online survey, and 80% reported no increased burden. A total of 60% of respondents said they had improved knowledge and had made clinical changes due to the study (see detailed comments in Anonymous Web-Based Survey Content found in the Supplemental Material).
Discussion
In this study, we show the potential of using the electronic health record systems to conduct a pragmatic, randomized clinical trial embedded in primary care, including identification of eligible patients, intervention delivery, and outcome ascertainment. We showed that willingness to participate in the study was high, and the study protocol did not increase provider burden. Despite these successes, we were unable to achieve the required between-arm differences in CKD testing rates to determine whether screening for CKD can improve clinical care for persons with hypertension.
We found that one in five veterans with hypertension and no diabetes who were tested had undetected CKD. Although albuminuria was the most common abnormality (51% identified by ACR only), the triple marker was required for accurate disease classification. In fact, >40% of patients with eGFRcreat <60 ml/min per 1.73 m2 were not confirmed by cystatin C or albuminuria, and these persons are considered low risk (4). Our findings also suggest that CKD detection has the potential to improve care because new use of RAS inhibitors was significantly higher among patients receiving the intervention compared with those receiving usual care in the as-treated analyses. A larger trial with protocol adjustments to increase testing rates is warranted to understand whether CKD screening improves clinical outcomes.
There is great interest in the implementation of pragmatic trials in nephrology (8). Our success in using the electronic health record for patient identification, enrollment, deployment of the intervention, and ascertainment of outcomes can serve as an important example. We were able to enroll >1800 participants in a short period of time, with a small study team, and within a limited budget. We enrolled older persons, up to 30% were nonwhite, and many had documented substance or alcohol use. We showed that the opt-out rate by patients was low (15). We were able to embed the intervention within the workflow of a primary care clinic, which resulted in high rates of participation by providers and a protocol that did not increase provider burden. The collaboration of a multidisciplinary team that leveraged an established health record system resulted in very thorough processes for data validation (15). We conclude that it is feasible to implement this protocol in any setting with an electronic health record and medical home model for primary care.
Despite the successes, we had some difficulties in the implementation of the protocol which taught important lessons. Less than half of participants randomized to intervention arms had a triple marker ordered, and this undermined our ability to achieve appropriate between-arm differences in CKD screening rates. This was due to the inability to detect appointments scheduled within a short time period. Because approximately 70% of persons with tests ordered completed testing, we expect that reach of the intervention would have been much higher with bulk orders or if we had worked with daily appointment data from the clinic sites directly. If we had added patient reminders to obtain laboratory tests, it may have increased testing rates, but would have reduced pragmatism. We also observed that few eligible patients had a pharmacist visit in the screen-educate plus pharmacist arm. We are unable to determine if this was due to lack of interest from the treating provider or lack of patient interest. An additional limitation is the possibility that a veteran used non-VA pharmacies for some medications. However, given that these veterans obtain regular primary care at the VA, this is unlikely to have been common (18). Our findings on the burden of the study are also limited because we could only survey attendings who remained in the clinic after study completion. Another barrier we encountered in this trial was potential crossover between arms. Patients of providers who left were reassigned to new or existing providers who may not have been randomized to the same study arm, potentially diluting differences between arms. For future trials, engagement of both patients and providers to understand willingness to perform all aspects of the intervention must be considered before deployment. Future studies may also need to incorporate repeat testing to confirm CKD status. In a future multisite trial, randomization by clinic rather than provider may alleviate the effects of provider changes.
An additional consideration relates to the consent requirement to send a letter and then wait 2 weeks for opting out, which limited our ability to increase the opportunity for testing. We believe that a rolling enrollment procedure can greatly increase intervention uptake, but this requires modified consent procedures. Human subjects and ethics teams must work with patients and investigators to find acceptable forms of consent that can facilitate learning health systems and implementation of low-risk interventions.
In summary, although we are unable to determine whether CKD screening followed by education and clinical guidance can improve care of veterans with hypertension, this study serves as an example and a foundation for pragmatic trials in nephrology that use the electronic health record to embed kidney-related interventions in primary care. Given that >20% of those tested had occult CKD, a larger study including primary care–embedded kidney support with appropriate multidisciplinary expertise, including nonphysician providers and information technology infrastructure, is warranted.
Data Sharing Statement
This trial has been registered on ClinicalTrials.gov and the required information has been submitted to the site. No identifiable data are available to the public. Study information is available at ClinicalTrials.gov per the National Institutes of Health policy.
Disclosures
Dr. Peralta is the Chief Medical Officer of Cricket Health and has ownership in the company. Dr. Shlipak reports that he is a scientific advisor for TAI Diagnostics and that he owns stock in the company. All other authors have nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R34DK102152 (Principal Investigator: Dr. Peralta). Dr. Peralta has received additional funding through the National Kidney Foundation and American Heart Association Established Investigator award 17EIA33410161.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05050419/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics for “as treated” population.
Supplemental Table 2. Exploratory analyses: New use of ACE/ARB, NSAIDs and diuretics, by study arm and intervention patients stratified by CKD status.
Anonymous web-based survey content.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Tuot DS, Plantinga LC, Hsu CY, Jordan R, Burrows NR, Hedgeman E, Yee J, Saran R, Powe NR; Centers for Disease Control Chronic Kidney Disease Surveillance Team : Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol 6: 1838–1844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group : KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl 3: 259–305, 2013 [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Moyer VA; U.S. Preventive Services Task Force : Screening for chronic kidney disease: U.S. Preventive services task force recommendation statement. Ann Intern Med 157: 567–570, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr.: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines [Published correction appears in Hypertension 71: e140–e144, 2018]. Hypertension 71: e13–e115, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D: Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA 305: 1545–1552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer IH, Kovesdy CP, Navaneethan SD, Peralta CA, Tuot DS, Vazquez MA, Crews DC; American Society of Nephrology Chronic Kidney Disease Advisory Group : Pragmatic clinical trials in CKD: Opportunities and challenges. J Am Soc Nephrol 27: 2948–2954, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooney D, Moon H, Liu Y, Miller RT, Perzynski A, Watts B, Drawz PE: A pharmacist based intervention to improve the care of patients with CKD: A pragmatic, randomized, controlled trial. BMC Nephrol 16: 56, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuot DS, McCulloch CE, Velasquez A, Schillinger D, Hsu CY, Handley M, Powe NR: Impact of a primary care CKD registry in a US public safety-net health care delivery system: A pragmatic randomized trial. Am J Kidney Dis 72: 168–177, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Nakhoul G, Konig V, Hyland J, Burrucker YK, Dann PD, Tucky BH, Sharp J, Nally JV: Pragmatic randomized, controlled trial of patient navigators and enhanced personal health records in CKD. Clin J Am Soc Nephrol 12: 1418–1427, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash DM, Ivers NM, Young J, Jaakkimainen RL, Garg AX, Tu K: Improving care for patients with or at risk for chronic kidney disease using electronic medical record interventions: A pragmatic cluster-randomized trial protocol. Can J Kidney Health Dis 4: 2054358117699833, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang AR, Evans M, Yule C, Bohn L, Young A, Lewis M, Graboski E, Gerdy B, Ehmann W, Brady J, Lawrence L, Antunes N, Green J, Snyder S, Kirchner HL, Grams M, Perkins R: Using pharmacists to improve risk stratification and management of stage 3A chronic kidney disease: A feasibility study. BMC Nephrol 17: 168, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dember LM, Lacson E Jr., Brunelli SM, Hsu JY, Cheung AK, Daugirdas JT, Greene T, Kovesdy CP, Miskulin DC, Thadhani RI, Winkelmayer WC, Ellenberg SS, Cifelli D, Madigan R, Young A, Angeletti M, Wingard RL, Kahn C, Nissenson AR, Maddux FW, Abbott KC, Landis JR: The TiME trial: A fully embedded, cluster-randomized, pragmatic trial of hemodialysis session duration. J Am Soc Nephrol 30: 890–903, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Frigaard M, Rubinsky AD, Rolon L, Lo L, Voora S, Seal K, Tuot D, Chao S, Lui K, Chiao P, Powe N, Shlipak M: Implementation of a pragmatic randomized trial of screening for chronic kidney disease to improve care among non-diabetic hypertensive veterans. BMC Nephrol 18: 132, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RE-AIM: Applying the RE-AIM Framework, 2019. Available at: http://www.re-aim.org/about/applying-the-re-aim-framework/. Accessed November 28, 2019
- 17.Austin PC, Stuart EA: Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34: 3661–3679, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroupe KT, Smith BM, Bailey L, Adas J, Gellad WF, Suda K, Huo Z, Tully S, Burk M, Cunningham F: Medication acquisition by veterans dually eligible for Veterans Affairs and Medicare Part D pharmacy benefits. Am J Health Syst Pharm 74: 140–150, 2017 [DOI] [PubMed] [Google Scholar]