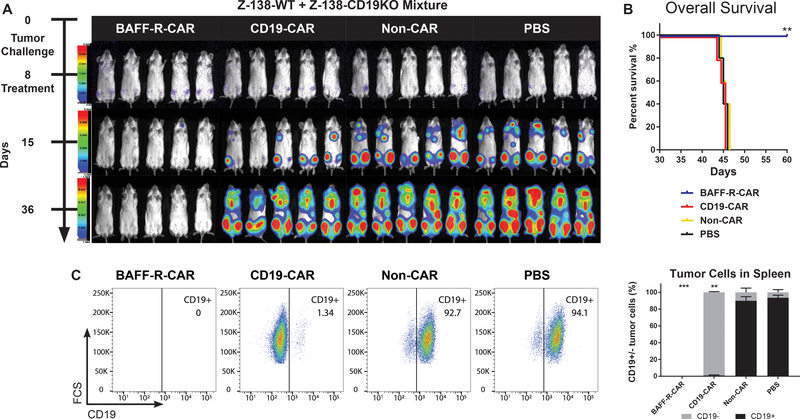
Figure 5. BAFF-R CAR T cells eliminate pre-existing CD19 antigen loss variants in vivo.
(a) Bioluminescence images of NSG mice following IV tumor challenge on day 0 with a mixture of 5 × 104 luciferase-expressing Z-138 (wildtype) plus 5 × 104 Z-138-CD19KO tumor cells. Groups of 5 tumor-bearing mice each were then randomly assigned to treatment with either 2.5 × 106 CD4 TN CAR-T + 106 CD8 TN BAFF-R- or CD19-CAR T cells/mouse IV on day 8, as a single dose. Non-transduced CD4/CD8 T cells from the same donor were used as allogeneic controls (non-CAR). (b) Kaplan-Meier plots of overall survival are shown. Log-rank test: **P<0.01 BAFF-R-CAR vs. CD19-CAR and controls. (c) Representative FACS plots of post-mortem tumor analysis from spleens of mice treated in (a). Cells were gated on CD45+ human tumor cells and analyzed for CD19 expression. Summary graph of mean percentage ± s.d. of triplicate samples CD19+/− tumor cells from n=5 mice/group. ***No tumor cells were detected. Two-way ANOVA and Tukey’s multiple comparisons test: **P<0.001 percentage of CD19-positive tumor cells vs. non-CAR and PBS controls.