FIGURE 2.
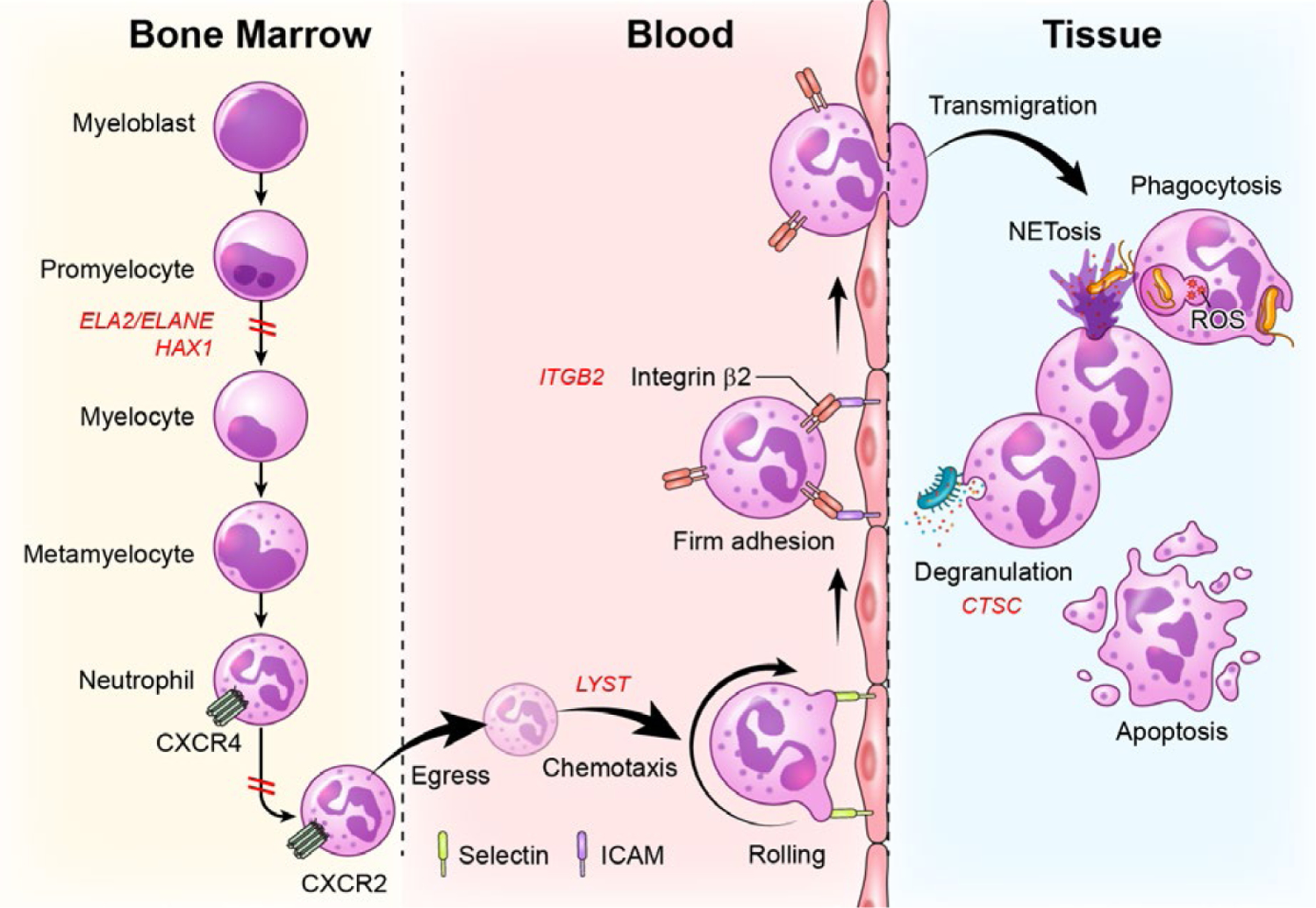
Defects in stages of neutrophil development, trafficking and function are linked to severe periodontitis. Neutrophils develop in the bone marrow in a process termed granulopoiesis. After progressing through discrete steps of maturation (myeloblast, promyelocytes, myelocyte, metamyelocyte, neutrophil), mature neutrophils will downregulate the expression of the chemokine CXCR4 and upregulate CXCR2 to exit the bone marrow via a process termed “egress”. Neutrophils will thereafter follow a chemokine gradient (chemotaxis) and will be led to tissue sites. For entry into tissues, neutrophils will follow discrete steps of tethering and rolling (through interaction with selectins) at the endothelial surface and firm adhesion through β2 integrin-ICAM interactions, which will allow extravasation/transmigration into tissues. In tissues, neutrophils will perform multiple functions, including microbe phagocytosis, degranulation, formation of nuclear extracellular traps (NETs) and will eventually die typically through apoptosis. Genetic defects at all of these stages of development, trafficking and function have been linked to periodontitis. Defects in neutrophil development due to mutations in ELA2 and/or HAX1are linked to severe congenital neutropenia and periodontitis. Defects in neutrophil egress due to gain of function mutations in CXCR4 are linked to WHIM syndrome and periodontitis. Defects in chemotaxis and diapedesis are linked to mutations in LYST in the syndrome Chediak-Higashi. Defects in the process of transmigration into tissues are due to mutations in ITGB2 which disrupts β2 integrin formation leading to Leukocyte Adhesion Deficiency-I and related periodontitis. Finally, defects in the activation and secretion of serine proteases (degranulation) are due to mutations in cysteine protease cathepsin C (CTSC), in the syndrome, Papillon Lefevre, which also predisposes to severe periodontitis at an early age
