Abstract
Functional trait-based approaches are increasingly adopted to understand and project ecological responses to environmental change; however, most assume trait expression is constant between conspecifics irrespective of context. Using two species of benthic invertebrate (brittlestars Amphiura filiformis and Amphiura chiajei), we demonstrate that trait expression at individual and community levels differs with biotic and abiotic context. We use PERMANOVA to test the effect of species identity, density and local environmental history on individual (righting and burrowing) and community (particle reworking and burrow ventilation) trait expression, as well as associated effects on ecosystem functioning (sediment nutrient release). Trait expression differs with context, with repercussions for the faunal mediation of ecosystem processes; we find increased rates of righting and burial behaviour and greater particle reworking with increasing density that are reflected in nutrient generation. However, the magnitude of effects differed within and between species, arising from site-specific environmental and morphological differences. Our results indicate that traits and processes influencing change in ecosystem functioning are products of both prevailing and historic conditions that cannot be constrained within typologies. Trait-based study must incorporate context-dependent variation, including intraspecific differences from individual to ecosystem scales, to avoid jeopardizing projections of ecosystem functioning and service delivery.
Keywords: bioturbation, community composition, functional diversity, functional traits, intraspecific variation, trait expression
1. Introduction
Decades of empirical study, motivated by unprecedented species loss and environmental change, have provided unequivocal evidence that altering biodiversity affects ecosystem functioning (e.g. primary production, nutrient cycling, sediment stability) and, ultimately, the provision of ecosystem services [1]. Current research emphasizes that rather than the number of species, ecosystem functioning is instead mediated by the functional traits (e.g. behavioural, morphological or life-history characteristics) expressed within a community [2,3]. As a result, functional trait-based approaches are increasingly adopted as predictive tools by ecosystem managers [4,5] as they incorporate species performance into projections of environmental change. In doing so, they confer understanding of the biological mechanisms underpinning faunal mediation of ecosystem functioning [6–8].
Conventional trait-based approaches and proposed frameworks implicitly assume that the expression of traits remains constant between conspecifics, irrespective of biotic or environmental context [9,10]. Studies may neglect intraspecific variability out of economic or logistical necessity, as measuring individual trait values in situ is not always possible. For management purposes, therefore, authors may rely on trait values from the literature or databases to characterize the functional importance of species [5,11,12]. In these approaches, the quantification of trait values and allocation of species to functional groups is frequently based on single mean trait values per species, and does not account for the scope and importance of intraspecific trait variability [13,14]. If the type or value of traits expressed are understood to determine a species' role in the ecosystem [15], any intraspecific variation potentially alters its contributions to ecosystem functioning and renders conventional typologies unsuitable.
Individual organisms are non-identical, with differing forms of trait expression distributed unevenly throughout communities [16,17]. It has long been appreciated that age classes, ontogenetic stages or sexes make differing contributions to ecosystem functioning. For example, individuals within a population are often grouped as being agender, despite knowledge that differing sexes can exhibit strongly distinct life strategies and energetic or resource demands [18]. Such physiological differences, including associated morphological differences in the mean and variance of body size, determine the scale of an individual's contribution to ecosystem functioning [19,20]. However, intraspecific variation occurs beyond demographic influences [21]. Some site-specific differences originate as a genetic component, stemming from long-term adaptation to historic conditions that creates distinct genetic ecotypes through multi-generational selection processes [22,23]. In addition, variation also arises over shorter temporal scales in the form of acclimation responses to prevailing biotic and abiotic conditions [15].
Mechanisms of phenotypic plasticity result in widespread and often substantial trait variability over time and space [17]. Transient trait expression in individuals alters their activities and potential contributions to ecosystem processes in response to habitat features [3,24], climatic drivers [25–28] and resource availability [29,30]. Incorporating the context-dependency of trait expression is vital for accuracy in the increasingly urgent quantification of ecosystem functioning under changing abiotic conditions [27]. Trait expression, furthermore, also shifts dramatically in response to biotic influences, primarily from neighbouring individuals and/or species [8,15,29,31]. Competitive or complementary interactions determine species coexistence and exclusion [32,33], and so potentially facilitate enhanced productivity, ecosystem functioning and service delivery [13]. Within species, local density-dependent effects can influence the expression of movement and life-history traits, influencing habitat use as conspecifics specialize behaviourally or physiologically to exploit available space and resources [34] or escape predation [35]. It is increasingly recognized that intraspecific differences in trait expression are not only widespread but also form an important component of biodiversity [14]. The representation of species using single or average trait values may fail to quantify responses to numerous aspects of ecological and environmental context [3], jeopardizing the reliability of approaches to ecosystem study and management [36,37].
In this study, we investigate the importance of incorporating intraspecific and individual-level trait variation into trait-based study, illustrating that faunally mediated community processes and ecosystem functioning with which these traits are associated are subject to context-dependent change. To achieve these aims, we interrogate the effect of biotic context and differing abiotic history on communities of two co-occurring species of infaunal marine invertebrate (brittlestars Amphiura filiformis and Amphiura chiajei). We hypothesize that (i) biotic and site-specific environmental context influence the expression of individual traits and community-level behaviour and that (ii) this variability would aid in understanding concurrent differences in biogeochemical proxies (nutrient concentration) for ecosystem function. To this effect, our results show that, contrary to the assumptions of prevailing trait-based modelling approaches, the trait expression and subsequent functional contributions of conspecific individuals cannot be assumed to be constant.
2. Material and methods
(a). Species collection and experimental design
Two species of ophiuroid brittlestars (A. filiformis and A. chiajei) were collected from two proximate sea lochs; Kilmaronag Shoal, Loch Etive (56°27′34.20″ N, 5°20′29.28″ W) and the Lynn of Lorne, Loch Linnhe (56°29′49.6″ N, 5°29′56.2″ W), Scotland, UK (electronic supplementary material, figure S1). Taxa with pelagic larvae, such as these species, have substantial distribution potential and are exchanged across landscape-scale distances and hydrographical barriers only in these early ontogenetic stages [23,38]. Given the proximate distance (approx. 12 km) and presence of substantial changes in seabed terrain and flow conditions between sites [39,40], we infer that individuals from each site are probably not genetically distinct but will have been exposed throughout their post-larval lifetimes to differing ecological and environmental conditions [41]. Loch Etive is subject to greater stratification and more frequent episodic flushing relative to Loch Linnhe that affects nutrient and organic material dynamics [40]. Sediment at Loch Etive is finer and contains a significantly higher total organic carbon (TOC) content in comparison to the Loch Linnhe site (ANOVA: F2,10 = 30.78, p < 0.001, electronic supplementary material, table S1 and figures S2 and S3).
Individuals were returned to the University of Southampton in aerated water baths and acclimated to aquarium conditions (approx. 12.6° C, 12 h L : 12 h D cycle, continually aerated) for a 30 day period. Estuarine mud from Hamble-le-Rice, Hampshire (50°52′23.1″ N 1°18′49.3″ W), was sieved (500 µm mesh) in a seawater bath to retain the fine fraction and remove macrofauna and allowed to settle for 48 h before being homogenized and distributed to Perspex aquaria (internal dimensions, L × W × H: 12 × 12 × 35 cm; settled depth approx. 10 cm overlaid with approx. 20 cm depth seawater, salinity 33). After 24 h and prior to the addition of the organisms, the seawater was replaced to remove excess dissolved nutrients associated with mesocosm assembly.
Our experiment required 102 aquaria arranged in a full factorial design (electronic supplementary material, tables S2 and S3). Replicate faunal assemblages (hereafter referred to as ‘communities’) from each sampling site (two levels; Loch Etive and Loch Linnhe, which represent historic exposures to discrete abiotic conditions hereafter referred to as ‘populations’) contained A. filiformis and A. chiajei in one of three species treatments (three levels; monoculture of A. filiformis, monoculture of A. chiajei or both species in mixture), across three naturally observed densities (three levels; low, medium and high, between 250 and 1000 ind m–2; electronic supplementary material, table S3). These species were selected for use, given their close taxonomic relationship, their shared tolerance for variable biotic and abiotic contexts [31] and their widespread co-occurrence throughout European shelf waters [39] where they exert a dominant influence on local biochemical cycling [42]. The three density levels manipulated span the range reported from across their European distribution [43–45] and therefore are not location-specific. For this study, we adjusted the densities of both species to reflect the approximate 3 A. filiformis : 2 A. chiajei ratio observed at the sample sites only as to avoid introducing novel aspects of biotic context. Each combination of factors was replicated six times, with the exception of two treatments (n = 4 and n = 5) (total n = 102; electronic supplementary material, table S3).
(b). Measures of individual trait expression
Individual-level behavioural trait expression was represented through movement and burial behaviours measured at the sediment surface following incubation and the quantification of community and ecosystem properties. Individuals were inverted and placed on the sediment surface in a temperature-controlled tray of sediment (3 cm depth overlaid with 5 cm depth seawater) under the same density and species treatment (monoculture or mixed) conditions in which they had been previously maintained. A benchtop video camera (uEYE USB camera, 1.3 MP, 25 FPS; IDS Imaging Development Systems, Obersulm, Germany) was used to record two righting and burial behaviours: (i) the time taken for each individual to begin movement activity, a response trait, and (ii) the time taken for each individual to right itself and bury fully into the sediment, an effect trait. Behaviour at the sediment surface reflects the strength and nature of organismal responses to their biological and physical surroundings [35], and burial rate is indicative of functionally relevant movement behaviours at the individual level [26].
As morphological traits can significantly influence an individual's functional contribution [19], we determined arm length (cm) and disc diameter (cm) using image analysis (ImageJ, v. 1.46r; [46]; electronic supplementary material, figure S4), and biomass (g), for each individual. Given the strong co-linearity between the metrics (electronic supplementary material, figure S5), the mean arm length (mean length of all five arms for each individual, producing an individual-level morphological trait) was used to represent morphological trait expression owing to its greater relevance in brittlestar motility and feeding behaviours [35,47].
(c). Measures of community behaviour
Burrow ventilation behaviour (bioirrigation) was estimated from the relative change in water column concentrations of the inert tracer sodium bromide (NaBr, dissolved in 20 ml = approx. 5 mM aquaria−1), over an 8 h period (NaBr, dissolved in 20 ml = approx. 5 mM aquaria−1; Δ[Br−], mg l−1); negative values indicate increased activity [48]. Filtered water samples (5 ml, 0.45 µm cellulose acetate membrane filter) were taken on day 29 of the experimental period and stored at 6°C prior to colorimetric analysis (FIAstar 5000 flow injection analyser, FOSS Tecator).
Faunally mediated particle reworking (bioturbation) was estimated non-invasively using sediment profile imaging (f-SPI) [49]. To visualize particle movement, 24 g dry weight aquaria−1 of dyed sediment that fluoresces in ultraviolet (UV) light (green colour; less than 125 µm; Brianclegg Ltd, UK) was introduced to the sediment surface on day 23 and imaged 8 days later (day 31). This length of time is sufficient to allow visualization of particle movement while avoiding vertical homogenization of the tracers. Images of all four sides of each mesocosm were taken within a UV illuminated imaging box. Following Solan et al. [49], images were saved in red-green-blue colour mode with JPEG compression and analysed using a custom-made semi-automated macro that runs within ImageJ (v. 1.46r), a Java-based public domain program [46]. From these data, the maximum depth of particle reworking (f-SPILmax) was calculated and surficial activity was estimated by quantifying surface boundary roughness (SBR), which is the maximum vertical deviation of the sediment–water interface (upper–lower limit; [50]).
(d). Measures of ecosystem functioning
Ecosystem functioning was represented through the proxy of sediment nutrient release, which is mediated by the sediment movement behaviours of benthic fauna [15,51]. Nutrient concentrations (ammonium, NH4-N; nitrate, NO3-N; nitrite, NO2-N; and phosphate, PO4-P; μmol l−1) were determined from filtered water samples (20 ml, Fisherbrand, nylon 0.45 µm, ⌀ 25 mm) taken on the final day of the experiment (day 30). Samples were frozen (−18°C) and analysed using a segmented flow autoanalyser (QuAAtro39 AutoAnalyzer).
(e). Statistical analysis
Permutational multivariate analysis of variance (PERMANOVA) and ANOVA were used to determine the independent and interacting effects of population (two levels; Loch Etive, Loch Linnhe), density (three levels; low, medium, high) and species identity (for intraspecific trait expression, four levels; A. filiformis in monoculture, A. filiformis in mixture, A. chiajei in monoculture, A. chiajei in mixture) or species mixture treatment (for community and ecosystem measures, three levels; A. filiformis monoculture, A. chiajei in monoculture, A. filiformis–A. chiajei mixed treatment) on individual and community behavioural trait expression, and associated ecosystem function. All statistical analyses were performed using the R statistical and programming environment [52] and the vegan package [53].
(i). Individual trait expression
Multivariate analyses were used to represent overall differences in the behavioural ‘personalities’ of individuals between species identities and contexts [54], integrating response (time to begin movement) and effect (time to complete burial) traits. PERMANOVA (iterations = 999) was used, as it is robust to non-normality and differing correlation structures and so is particularly suited for the detection of differences in intraspecific trait expression [55]. Patterns of intraspecific trait expression differ between the behavioural traits, and between context treatments (electronic supplementary material, figure S6). Permutational analysis of multivariate dispersion (PERMDISP) was used to test for homogeneity of variance between populations (F1,190 = 0.57, p = 0.45), species identities (F1,188 = 1.20, p = 0.31) and densities (F1,189 = 1.22, p = 0.30). These results support that any significant differences in PERMANOVA between treatments are owing to changes in the values of trait expression, not shifts in the overall extent of variation itself. Nevertheless, to negate any dispersion effects caused by unequal numbers of individuals between groups, we standardized abundance between species treatments and density levels (n = 192) (electronic supplementary material, table S3).
PERMANOVA models were developed to test the independent and interacting effects of: (i) community-level effects (population, species identity, density) and (ii) individual-level differences in morphological trait expression (mean arm length) between communities (population, species identity), on multivariate intraspecific behavioural trait expression. Data exploration showed there were differences in morphological trait expression between populations (ANOVA: F1,188 = 4.03, p = 0.046) and species (ANOVA: F1,188 = 14.99, p < 0.001) which may contribute to observed site-specific and interspecific effects.
To quantify the extent of intraspecific trait variation, the coefficient of variation (CV; the ratio of standard deviation to the mean) was determined for the expression of each individual-level trait (time to begin movement, time to complete burial and mean arm length).
(ii). Community behaviour and ecosystem functioning
Four-way ANOVA was used to test the independent and interactive effects of context (population, species treatment, density) and intraspecific variation in morphological trait expression (CV of mean arm length) on each community-level behaviour (Δ[Br−], f-SPILmax, SBR), and a three-way ANOVA was used to test the independent and interactive effects of context (population, species treatment, density) on nutrient concentration ([NH4-N], [NO3-N], [NO2-N], [PO4-P]). Model assumptions were assessed visually for normality (Q–Q plot), heterogeneity of variance (plotted residuals versus fitted values), and the presence of outliers or overly influential data points (Cook's distance) and the minimal adequate effects structure was determined using backward selection informed by Akaike information criteria [56].
3. Results
(a). Individual trait expression
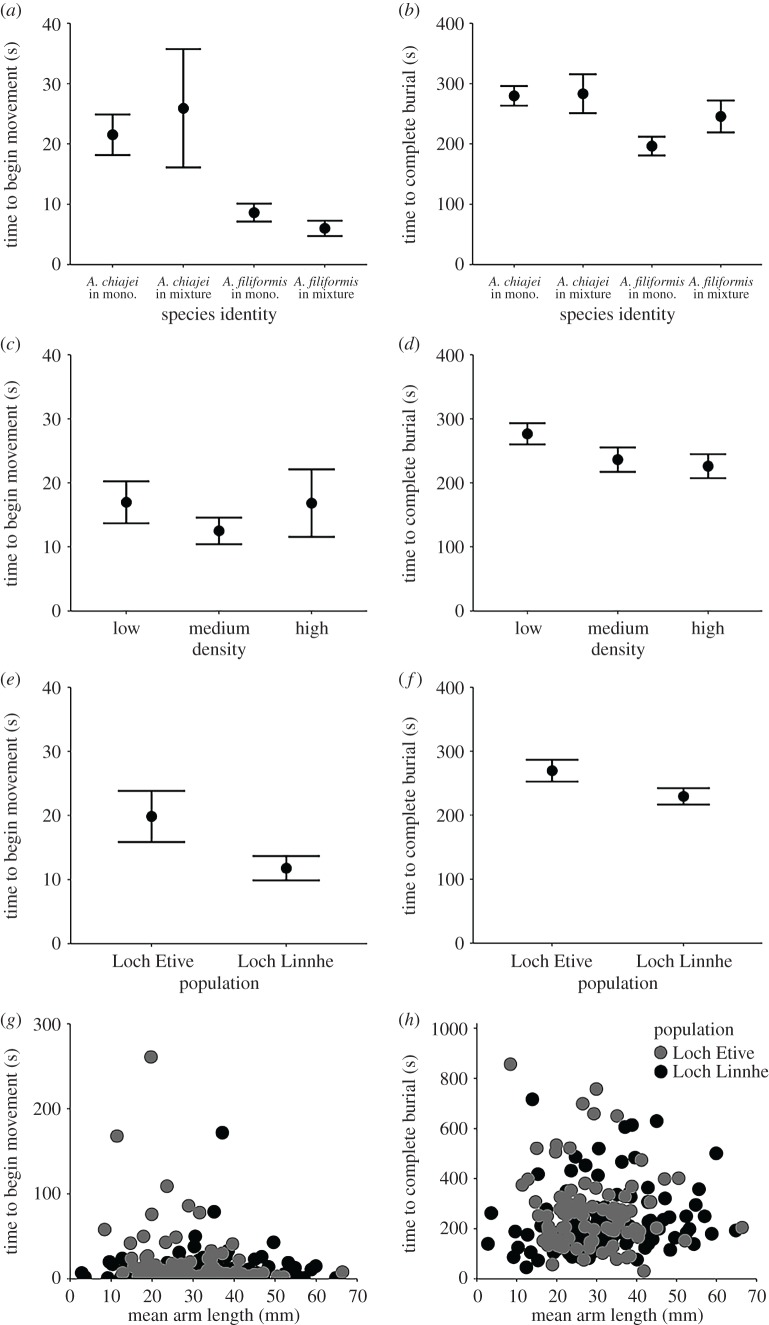
With respect to all aspects of context (population, species identity, density), PERMANOVA revealed that behavioural trait expression was dependent on the independent effects of species identity (F3,168 = 6.08, p < 0.01), density (F2,168 = 3.82, p < 0.01) and population (F1,168 = 4.24, p = 0.025) (figure 1a–f).
Figure 1.
The effect of biotic and abiotic context on time elapsed (mean ± s.e.) (s) for A. chiajei and A. filiformis to (a,c,e,g) begin movement and (b,d,f,h) complete burial into the sediment, where (a,b) show the independent effects of species identity, (c,d) show the independent effect of density, (e,f) show the independent effect of population of origin and (g,h) show the interactive effect of population × mean arm length (mm).
When considered alongside only those aspects of context which define the identity (population, species identity) and morphological trait expression (mean arm length) of individuals, behavioural trait expression was dependent on the interactive effects of mean arm length × population of origin (PERMANOVA: F1,176 = 3.71, p = 0.036) (figure 1e–h), in addition to the independent effect of species identity (PERMANOVA: F3,176 = 5.72, p < 0.01) (figure 1a,b).
Though analysed together in a multivariate manner, both movement behaviours (time to begin activity and time to complete burial) were visualized independently to highlight differences in expression between each trait. Overall, A. chiajei took significantly longer before beginning or completing burial than A. filiformis; however, intraspecific differences are present in both species and between individuals maintained in monoculture or in a mixed community. The extent of these context-dependent differences varied depending on the trait, and patterns between treatment conditions were consistently less prominent for the time taken to begin movement. For both A. chiajei and A. filiformis, the time taken to fully complete burial was increased in mixed species treatments in comparison to monoculture, with a similar if weaker pattern suggested for A. chiajei and the time taken to begin movement (figure 1a,b). For both species, the time taken to complete burial decreased with density (figure 1c,d). Individuals from Loch Linnhe had significantly (ANOVA: F1,188 = 4.033, p = 0.046) larger mean arm lengths (A. filiformis mean ± s.e. (n = 55) 27.88 ± 11.17, A. chiajei mean ± s.e. (n = 51) 36.54 ± 12.85; electronic supplementary material, figure S7) than those originating from Loch Etive (A. filiformis mean ± s.e. (n = 40) 27.06 ± 7.57, A. chiajei mean ± s.e. (n = 46) 30.30 ± 11.68), and completed movement behaviours more rapidly (figure 1e–h). The coefficient of variation of both behavioural traits (time to begin activity and time to complete burial) within communities did not differ significantly between variables or their interactions (ANOVA: p > 0.05 for all, electronic supplementary material, table S4), though trends suggest comparatively greater extents of variation may occur for both behavioural traits for individuals maintained under elevated density or in a mixed species treatment, or those originating from Loch Etive (electronic supplementary material, figure S8).
(b). Community behaviour
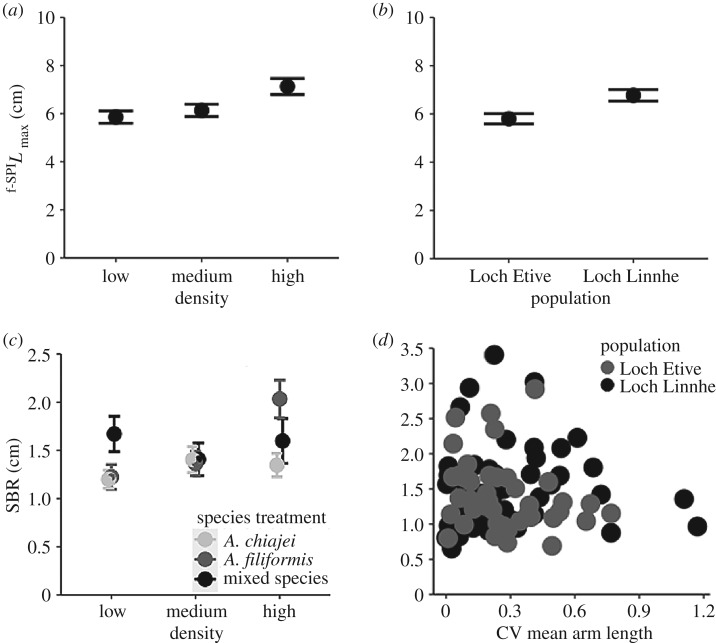
Community-level bioturbation and bioirrigation behaviours were differentially affected by abiotic and biotic context (species mixture treatment, density, population) and morphological trait variation. The maximum depth of particle redistribution, f-SPILmax, was significantly affected by the independent effects of density (ANOVA: F2,60 = 5.85, p < 0.01) and population (ANOVA: F1,60 = 8.68, p < 0.01). f-SPILmax increased with density (figure 2a), while remaining shallower in mesocosms with individuals from Loch Etive in comparison to Loch Linnhe (coefficient ± s.e. = 0.40 ± 0.51, t = 0.78, p = 0.44) (figure 2b). SBR differed significantly with the interactive effects of density × species treatment (ANOVA: F4,74 = 3.16, p = 0.018), and population of origin × morphological trait variation (ANOVA: F1,74 = 4.81, p = 0.031). The magnitude of differences in SBR between species treatments were increased at greater densities (figure 2c), with higher SBR found in Loch Linnhe communities with greater morphological trait variation (CV mean arm length) (figure 2d). Though the extent of variation for average arm length did not differ significantly between densities (ANOVA: F2,78 = 1.76, p = 0.18), species treatments (ANOVA: F2,78 = 0.61, p = 0.55) or populations (ANOVA: F1,78 = 0.02, p = 0.88), variation in the morphology of individuals was comparatively elevated for individuals originating from Loch Linnhe or maintained under medium density (electronic supplementary material, figure S9). Bioirrigation activity (Δ[Br−]) did not vary with abiotic or biotic context as results showed that, although the density × population interaction was included in the minimal adequate mode, its effects were non-significant (ANOVA: F2,90 = 1.11, p = 0.34, electronic supplementary material, figure S10).
Figure 2.
The effects of biotic and abiotic context on (mean ± s.e., n = 6) (a,b) f-SPILmax (cm) and (c,d) SBR (cm) in mesocosms containing A. chiajei and A. filiformis in monoculture or mixture, showing the (a) independent effect of density and (b) the independent effect of population, (c) the interactive effect of density × species treatment, and (d) the interactive effect of morphological trait variation (CV of mean arm length) and population.
(c). Ecosystem functioning
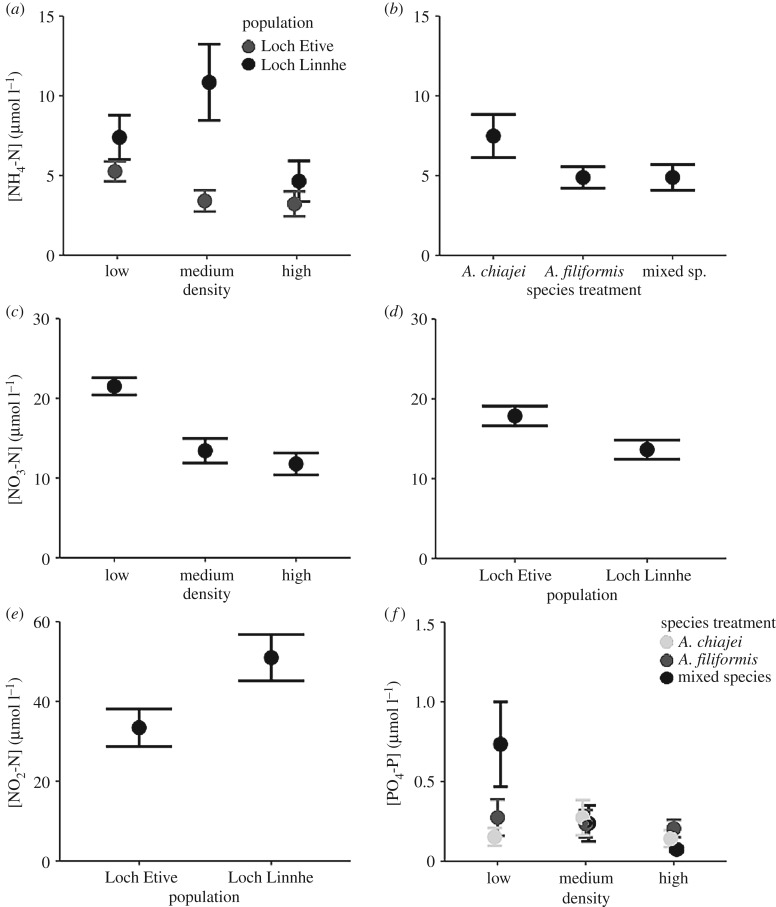
The effect of biotic and abiotic context on sediment nutrient release differed between nutrients (figure 3). [NH4-N] was significantly influenced by the interactive effect of population × density (ANOVA: F2,85 = 3.15, p = 0.048). Overall, [NH4-N] was increased in communities originating from Loch Linnhe in comparison to those from Loch Etive (coefficient ± s.e. = 2.31 ± 1.81, t = 1.27, p = 0.21), with clearer differences in [NH4-N] between populations at lower densities (figure 3a). [NH4-N] was also significantly affected by species treatment (ANOVA: F2,85 = 3.22, p = 0.045), being greatest in A. chiajei monoculture communities (figure 3b). [NO3-N] was significantly affected by density (ANOVA: F2,89 = 16.38, p < 0.01) and population (ANOVA: F1,89 = 6.95, p < 0.01), decreasing with density, and with lower concentrations found in Loch Linnhe communities (coefficient ± s.e. = −3.95 ± 1.5, t = −2.64, p < 0.01) (figure 3c,d). [NO2-N] was significantly affected by population (ANOVA: F1,83 = 5.94, p = 0.017), showing greater concentrations in communities originating from Loch Linnhe (coefficient ± s.e. = 17.83 ± 7.43, t = 2.4, p = 0.019) (figure 3e). [PO4-P] was significantly affected by the interactive effect of species treatment × density (ANOVA: F4,84 = 2.81, p = 0.030), with overall PO4-P concentration, and the magnitude of difference between species treatments, decreasing with density (figure 3f).
Figure 3.
The effects of differing biotic and abiotic context on (mean ± s.e., n = 6) (µmol l−1) (a,b) [NH4-N], (c,d) [NO3-N], (e) [NO2-N] and (f) [PO4-P] where (a) shows the interactive effects of density × population, (b) shows the independent effect of species treatment, (c,d) show the independent effects of density and population, respectively, (e) shows the independent effect of population and (f) the interactive effect of density × species treatment.
4. Discussion
Overall, our results demonstrate significant influence of context on the trait expression of individuals. We show that this context-dependency then affects the functional roles and contributions of species by mechanistically underpinning concurrent change in community behaviour and ecosystem functioning.
We found site-specific and interspecific differences in morphological trait expression. By consequence, it is difficult to interrogate the role of population or species per se in determining behavioural trait expression. Body size determines the scaling relationship between the traits expressed by a species and their ecosystem role, and larger individuals are often liable to have stronger effects on ecosystem functioning [57]. Given this relationship, intraspecific morphological variability has already been incorporated into some functional trait approaches via a community average [49]. Body size traits are a complex and potentially transient response to genetic influences, age, food and other resources [58]. Even where two organisms are allegedly found within the same functional group, larger individuals are expected to have proportionally larger effects to ecosystem functioning (e.g. displace more sediment and pump more water [19]), and intraspecific morphological expression may be a significant influence on the functional roles of species. However, even beyond the contributions of morphological differences, individuals with shared local histories are likely to consistently express similar traits [28,54,59]. Abiotic context influences the presence, plasticity and strength of traits expressed within a community [22,24,26]. Organic matter content and sediment grain size, which differ between Loch Etive and Loch Linnhe, notably affect organism behaviour in terms of sediment mixing and bioirrigation [60,61]. Origin in the distinct conditions of either loch contributes to differences in trait expression at an individual level, and in the community-level net effects which these traits in part underpin [15].
Further, density and species identity influence intraspecific behavioural trait expression as community composition determines the neighbour-effects that dictate behaviours including space and resource use [31,34,62]. These effects in turn underpin the role of shifting biodiversity in driving altered ecosystem functioning [8]. Changes in the extent and structure of biodiversity alter not only functional diversity at the community level, but form differing biotic contexts with influence on the trait expression and functional roles of component individuals [1,15]. Behavioural factors are among the more flexible aspects of an animal's phenotype as they are less likely to be constrained by strict physiological tolerances, and so their variation readily reflects short- and long-term responses of each species to local conditions [59]. The competitive advantage offered by this trait dissimilarity, and so its role in determining community structure, depends on whether individuals are involved in intra- and interspecific competition, as species may benefit from expressing novel [13] or more acquisitive phenotypes [37]. Our results show that, even where species are distinguished by interspecific differences in behavioural or morphological traits [63], each taxa may also display distinct intraspecific responses between communities of differing compositions [64].
The potential for intraspecific variation should not be overlooked, given that it can strongly determine the functional identity and context-dependent contributions of each species [14]. Context-dependent variation may have consequences for ecosystem functioning as it can change, expand or narrow the distribution of relevant traits expressed and so alter the assumed functional contributions of organisms [65]. Differences in sediment reworking between treatments mechanistically underpin the differences in dissolved nutrient release observed between the same conditions, demonstrating that change in behavioural trait expression influences biogeochemical processes and so mediates the functioning of benthic habitats [15,51]. However, establishing the relative importance of intraspecific and interspecific variation has long been a focus of trait-based ecology [9,64]. The necessity of considering intraspecific variation is likely to be determined by the extent of variability within a trait [66], the strength of its relationship with ecosystem function [67] and indeed the research question at hand. We suggest that quantifying the extent of intraspecific variation should be a particular priority where environmental conditions are changing, or where taxa are compared across gradients. Mesocosm experimental studies or subsampling of trait expression in situ offers ability to establish the realized functional contributions or variability of species in complement to conventional trait-based study [66]. It is probable that interspecific differences will exceed intraspecific differences in terms of magnitude [68], and that quantification of intraspecific variability will be less likely to alter projections of functioning and service delivery at ecosystem scales with high species richness [69]. Nonetheless, to do so characterizes the sources, pathways and potential consequences of altered conditions [9,59]. Intraspecific trait variation and its covariation with interspecific trait variation together determine community responses to ecological change [64].
Given that natural systems are increasingly subject to drivers of ecological change, we highlight the need to determine the contexts in which intraspecific variability arises [54,65]. Within this framework, we must isolate the circumstances where it contributes to the functional integrity of ecosystems [64,69]. Failure to do so jeopardizes understanding and prediction of ecosystem functioning owing to inadequate characterization of traits and, by result, biodiversity [1,14,15]. Trait-based models for predicting community structure across environmental gradients perform poorly when they fail to integrate the effects of intraspecific variation in functional traits, as existing typologies are insufficiently broad [3]. Our findings demonstrate that trait-based approaches to ecosystem study require more detailed functional metrics than has previously been assumed. Future efforts should seek to report responses under multiple ecosystem conditions, to demonstrate the potential breadth of resulting intraspecific diversity, and consider how these effects will propagate up biological scales [7,13,16,65].
5. Conclusion
Our findings show that the expression of traits by individuals and so the net behaviour of their communities differs with biotic and abiotic context. Such changes in individual functional contributions have important implications for mediation of ecosystem functioning. Our study highlights that trait-based approaches which do not consider the context-dependency of trait expression are at risk of misrepresenting the functional roles of taxa. The quantification of intraspecific variability will offer ecologists better insight into biological responses to environmental conditions, and aid ecosystem management approaches seeking to maintain good ecosystem function and service delivery in the face of environmental change.
Supplementary Material
Data accessibility
Supporting information for this article have been uploaded as the electronic supplementary material. Raw data are archived at the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.14t4h37 [70].
Authors' contributions
C.C., J.A.G., L.J.G., C.G. and S.G.B. conceived the study. C.C. and J.A.G. conducted fieldwork. C.C. constructed and maintained experiments, and completed data and statistical analyses. All coauthors contributed to the interpretation of results and manuscript production.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Natural Environment Research Council (grant no. NE/L002531/1) via a studentship to C.C. under the SPITFIRE DTP.
References
- 1.Adair EC, Hooper DU, Paquette A, Hungate BA. 2018. Ecosystem context illuminates conflicting roles of plant diversity in carbon storage. Ecol. Lett. 21, 1604–1619. ( 10.1111/ele.13145) [DOI] [PubMed] [Google Scholar]
- 2.Gagic V, et al. 2015. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices . Proc. R. Soc. B 282, 20142620 ( 10.1098/rspb.2014.2620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Read QD, Henning JA, Sanders NJ. 2017. Intraspecific variation in traits reduces ability of trait-based models to predict community structure. J. Veg. Sci. 28, 1070–1081. ( 10.1111/jvs.12555) [DOI] [Google Scholar]
- 4.Rijnsdorp AD, et al. 2015. Towards a framework for the quantitative assessment of trawling impact on the seabed and benthic ecosystem. ICES J. Mar. Sci. 73, i127–i138. ( 10.1093/icesjms/fsv207) [DOI] [Google Scholar]
- 5.Bolam SG, et al. 2017. Differences in biological traits composition of benthic assemblages between unimpacted habitats. Mar. Environ. Res. 126, 1–13. ( 10.1016/j.marenvres.2017.01.004) [DOI] [PubMed] [Google Scholar]
- 6.Laughlin DC. 2014. Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol. Lett. 17, 771–784. ( 10.1111/ele.12288) [DOI] [PubMed] [Google Scholar]
- 7.Funk JL, et al. 2017. Revisiting the Holy Grail: using plant functional traits to understand ecological processes. Biol. Rev. 92, 1156–1173. ( 10.1111/brv.12275) [DOI] [PubMed] [Google Scholar]
- 8.Thomsen MS, Godbold JA, Garcia C, Bolam SG, Parker R, Solan M. 2019. Compensatory responses can alter the form of the biodiversity–function relation curve. Proc. R. Soc. B 286, 20190287 ( 10.1098/rspb.2019.0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S. 2010. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct. Ecol. 24, 1192–1201. ( 10.1111/j.1365-2435.2010.01727.x) [DOI] [Google Scholar]
- 10.Hevia V, Martín-López B, Palomo S, García-Llorente M, Bello F, González JA. 2017. Trait-based approaches to analyze links between the drivers of change and ecosystem services: synthesizing existing evidence and future challenges. Ecol. Evol. 7, 831–844. ( 10.1002/ece3.2692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gogina M, et al. 2016. The Baltic Sea scale inventory of benthic faunal communities. ICES J. Mar. Sci. 73, 1196–1213. ( 10.1093/icesjms/fsv265) [DOI] [Google Scholar]
- 12.Solan M, Ward ER, White EL, Hibberd EE, Cassidy C, Schuster JM, Hale R, Godbold JA. 2019. Worldwide measurements of bioturbation intensity, ventilation rate, and the mixing depth of marine sediments. Sci. Data 6, 58 ( 10.1038/s41597-019-0069-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finerty GE, de Bello F, Bílá K, Berg MP, Dias AT, Pezzatti GB, Moretti M. 2016. Exotic or not, leaf trait dissimilarity modulates the effect of dominant species on mixed litter decomposition. J. Ecol. 104, 1400–1409. ( 10.1111/1365-2745.12602) [DOI] [Google Scholar]
- 14.Des Roches S, Post DM, Turley NE, Bailey JK, Hendry AP, Kinnison MT, Schweitzer JA, Palkovacs EP. 2018. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2, 57–64. ( 10.1038/s41559-017-0402-5) [DOI] [PubMed] [Google Scholar]
- 15.Wohlgemuth D, Solan M, Godbold JA. 2017. Species contributions to ecosystem process and function can be population dependent and modified by biotic and abiotic setting. Proc. R. Soc. B 284, 20162805 ( 10.1098/rspb.2016.2805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona CP, de Bello F, Mason NW, Lepš J. 2016. Traits without borders: integrating functional diversity across scales. Trends Ecol. Evol. 31, 382–394. ( 10.1016/j.tree.2016.02.003) [DOI] [PubMed] [Google Scholar]
- 17.Roscher C, Gubsch M, Lipowsky A, Schumacher J, Weigelt A, Buchmann N, Schulze E-D, Schmid B. 2018. Trait means, trait plasticity and trait differences to other species jointly explain species performances in grasslands of varying diversity. Oikos 127, 865 ( 10.1111/oik.04815) [DOI] [Google Scholar]
- 18.Rudolf VH, Rasmussen NL. 2013. Population structure determines functional differences among species and ecosystem processes. Nat. Commun. 4, 2318 ( 10.1038/ncomms3318) [DOI] [PubMed] [Google Scholar]
- 19.Norkko A, Villnäs A, Norkko J, Valanko S, Pilditch C. 2013. Size matters: implications of the loss of large individuals for ecosystem function. Sci. Rep. 3, 2646 ( 10.1038/srep02646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritschie KJ, Olden JD. 2016. Disentangling the influences of mean body size and size structure on ecosystem functioning: an example of nutrient recycling by a non-native crayfish. Ecol. Evol. 6, 159–169. ( 10.1002/ece3.1852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell RM, Bakker JD. 2014. Intraspecific trait variation driven by plasticity and ontogeny in Hypochaeris radicata. PLoS ONE 9, e109870 ( 10.1371/journal.pone.0109870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calosi P, et al. 2013. Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Phil. Trans. R. Soc. B 368, 20120444 ( 10.1098/rstb.2012.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins PE, Neill SP, Giménez L, Jenkins SR, Malham SK. 2013. Physical and biological controls on larval dispersal and connectivity in a highly energetic shelf sea. Limnol. Oceanogr. 58, 505–524. ( 10.4319/lo.2013.58.2.0505) [DOI] [Google Scholar]
- 24.Törnroos A, Nordström MC, Aarnio K, Bonsdorff E. 2015. Environmental context and trophic trait plasticity in a key species, the tellinid clam Macoma balthica L. J. Exp. Mar. Biol. Ecol. 472, 32–40. ( 10.1016/j.jembe.2015.06.015) [DOI] [Google Scholar]
- 25.Baranov V, Lewandowski J, Krause S. 2016. Bioturbation enhances the aerobic respiration of lake sediments in warming lakes. Biol. Lett. 12, 20160448 ( 10.1098/rsbl.2016.0448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagelkerken I, Munday PL. 2016. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob. Change Biol. 22, 974–989. ( 10.1111/gcb.13167) [DOI] [PubMed] [Google Scholar]
- 27.Landeira-Dabarca A, Pérez J, Graça MA, Boyero L. 2019. Joint effects of temperature and litter quality on detritivore-mediated breakdown in streams. Aquat. Sci. 81, 1 ( 10.1007/s00027-018-0598-8) [DOI] [Google Scholar]
- 28.Peterson ML, Doak DF, Morris WF. 2019. Incorporating local adaptation into forecasts of species' distribution and abundance under climate change. Glob. Change Biol. 25, 775–793. ( 10.1111/gcb.14562) [DOI] [PubMed] [Google Scholar]
- 29.Hawlena D, Hughes KM, Schmitz OJ. 2011. Trophic trait plasticity in response to changes in resource availability and predation risk. Funct. Ecol. 25, 1223–1231. ( 10.1111/j.1365-2435.2011.01891.x) [DOI] [Google Scholar]
- 30.Murray F, Solan M, Douglas A. 2017. Effects of algal enrichment and salinity on sediment particle reworking activity and associated nutrient generation mediated by the intertidal polychaete Hediste diversicolor. J. Exp. Mar. Biol. Ecol. 495, 75–82. ( 10.1016/j.jembe.2017.06.002) [DOI] [Google Scholar]
- 31.Calder-Potts R, Spicer JI, Calosi P, Findlay HS, Queiros AM, Widdicombe S. 2018. Density-dependent responses of the brittlestar Amphiura filiformis to moderate hypoxia and consequences for nutrient fluxes. Mar. Ecol. Prog. Ser. 594, 175–191. ( 10.3354/meps12503) [DOI] [Google Scholar]
- 32.Turcotte MM, Levine JM. 2016. Phenotypic plasticity and species coexistence. Trends Ecol. Evol. 31, 803–813. ( 10.1016/j.tree.2016.07.013) [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Ramos IM, Matías L, Gómez-Aparicio L, Godoy Ó. 2019. Functional traits and phenotypic plasticity modulate species coexistence across contrasting climatic conditions. Nat. Commun. 10, 2555 ( 10.1038/s41467-019-10453-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraft NJ, Godoy O, Levine JM. 2015. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl Acad. Sci. USA 112, 797–802. ( 10.1073/pnas.1413650112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg R, Selander E. 2000. Alarm signal response in the brittle star Amphiura filiformis . Mar. Biol. 136, 43–48. ( 10.1007/s002270050006) [DOI] [Google Scholar]
- 36.Reich PB, Rich RL, Lu X, Wang YP, Oleksyn J. 2014. Biogeographic variation in evergreen conifer needle longevity and impacts on boreal forest carbon cycle projections. Proc. Natl Acad. Sci. USA 111, 13 703–13 708. ( 10.1073/pnas.1216054110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett JA, Riibak K, Tamme R, Lewis RJ, Pärtel M. 2016. The reciprocal relationship between competition and intraspecific trait variation. J. Ecol. 104, 1410–1420. ( 10.1111/1365-2745.12614) [DOI] [Google Scholar]
- 38.Ershova E, Descoteaux R, Wangensteen O, Iken K, Hopcroft R, Smoot C, Grebmeier JM, Bluhm BA. 2019. Diversity and distribution of meroplanktonic larvae in the Pacific Arctic and connectivity with adult benthic invertebrate communities. Front. Mar. Sci. 6, 490 ( 10.3389/fmars.2019.00490) [DOI] [Google Scholar]
- 39.Gage J. 1972. A preliminary survey of the benthic macrofauna and sediments in Lochs Etive and Creran, sea-lochs along the west coast of Scotland. J. Mar. Biol. Assoc. UK 52, 237–276. ( 10.1017/S0025315400018683) [DOI] [Google Scholar]
- 40.Friedrich J, et al. 2014. Investigating hypoxia in aquatic environments: diverse approaches to addressing a complex phenomenon. Biogeosciences 11, 1215–1259. ( 10.5194/bg-11-1215-2014) [DOI] [Google Scholar]
- 41.Alp M, Keller I, Westram AM, Robinson CT. 2012. How river structure and biological traits influence gene flow: a population genetic study of two stream invertebrates with differing dispersal abilities. Freshwater Biol. 57, 969–981. ( 10.1111/j.1365-2427.2012.02758.x) [DOI] [Google Scholar]
- 42.Murray F, Widdicombe S, McNeill CL, Solan M. 2013. Consequences of a simulated rapid ocean acidification event for benthic ecosystem processes and functions. Mar. Pollut. Bull. 73, 435–442. ( 10.1016/j.marpolbul.2012.11.023) [DOI] [PubMed] [Google Scholar]
- 43.O'Connor B, Bowmer T, Grehan A. 1983. Long-term assessment of the population dynamics of Amphiura filiformis (Echinodermata: Ophiuroidea) in Galway Bay (west coast of Ireland). Mar. Biol. 75, 279–286. ( 10.1007/BF00406013) [DOI] [Google Scholar]
- 44.Duineveld GCA, Künitzer A, Heyman RP. 1987. Amphiura filiformis (Ophiuroidea: Echinodermata) in the North Sea. Distribution, present and former abundance and size composition. Neth. J. Sea Res. 21, 317–329. ( 10.1016/0077-7579(87)90006-8) [DOI] [Google Scholar]
- 45.Munday BW, Keegan BG. 1992. Population dynamics of Amphiura chiajei (Echinodermata: Ophiuroidea) in Killary Harbour, on the west coast of Ireland. Mar. Biol. 114, 595–605. ( 10.1007/BF00357256) [DOI] [Google Scholar]
- 46.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Astley HC. 2012. Getting around when you're round: quantitative analysis of the locomotion of the blunt-spined brittle star, Ophiocoma echinata. J. Exp. Biol. 215, 1923–1929. ( 10.1242/jeb.068460) [DOI] [PubMed] [Google Scholar]
- 48.Forster S, Glud RN, Gundersen J.K, Huettel M. 1999. In situ study of bromide tracer and oxygen flux in coastal sediments. Estuar. Coast. Shelf Sci. 49, 813–827. ( 10.1006/ecss.1999.0557) [DOI] [Google Scholar]
- 49.Solan M, Wigham BD, Hudson IR, Kennedy R, Coulon CH, Norling K, Nilsson HC, Rosenberg R. 2004. In situ quantification of bioturbation using time lapse fluorescent sediment profile imaging (f SPI), luminophore tracers and model simulation. Mar. Ecol. Prog. Ser. 271, 1–12. ( 10.3354/meps271001) [DOI] [Google Scholar]
- 50.Hale R, Mavrogordato MN, Tolhurst TJ, Solan M. 2014. Characterizations of how species mediate ecosystem properties require more comprehensive functional effect descriptors. Sci. Rep. 4, 6463 ( 10.1038/srep06463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kristensen E, Delefosse M, Quintana CO, Flindt MR, Valdemarsen T. 2014. Influence of benthic macrofauna community shifts on ecosystem functioning in shallow estuaries. Front. Mar. Sci. 1, 41 ( 10.3389/fmars.2014.00041) [DOI] [Google Scholar]
- 52.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 53.Oksanen J, et al. 2017. vegan: community ecology package. R package version 2.4-4. See https://CRAN.R-project.org/package=vegan.
- 54.Moran NP, Mossop KD, Thompson RM, Chapple DG, Wong BB. 2017. Rapid divergence of animal personality and syndrome structure across an arid-aquatic habitat matrix. Oecologia 185, 55–67. ( 10.1007/s00442-017-3924-2) [DOI] [PubMed] [Google Scholar]
- 55.Mitchell RM, Bakker JD. 2014. Quantifying and comparing intraspecific functional trait variability: a case study with Hypochaeris radicata. Funct. Ecol. 28, 258–269. ( 10.1111/1365-2435.12167) [DOI] [Google Scholar]
- 56.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 57.Larsen TH, Williams NM, Kremen C. 2005. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol. Lett. 8, 538–547. ( 10.1111/j.1461-0248.2005.00749.x) [DOI] [PubMed] [Google Scholar]
- 58.Liao WB, Luo Y, Lou SL, Lu D, Jehle R. 2016. Geographic variation in life-history traits: growth season affects age structure, egg size and clutch size in Andrew's toad (Bufo andrewsi). Front. Zool. 13, 6 ( 10.1186/s12983-016-0138-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher DN, David M, Tregenza T, Rodríguez-Muñoz R. 2015. Dynamics of among-individual behavioral variation over adult lifespan in a wild insect. Behav. Ecol. 26, 975–985. ( 10.1093/beheco/arv048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bulling MT, et al. 2008. Species effects on ecosystem processes are modified by faunal responses to habitat composition. Oecologia 158, 511–520. ( 10.1007/s00442-008-1160-5) [DOI] [PubMed] [Google Scholar]
- 61.Godbold JA, Solan M. 2009. Relative importance of biodiversity and the abiotic environment in mediating an ecosystem process. Mar. Ecol. Prog. Ser. 396, 273–282. ( 10.3354/meps08401) [DOI] [Google Scholar]
- 62.De Backer A, Coillie FV, Montserrat F, Provoost P, Colen CV, Vincx M, Degraer S. 2011. Bioturbation effects of Corophium volutator: importance of density and behavioural activity. Estuar. Coast. Shelf Sci. 91, 306–313. ( 10.1016/j.ecss.2010.10.031) [DOI] [Google Scholar]
- 63.Buchanan JB. 1964. A comparative study of some features of the biology of Amphiura filiformis and Amphiura chiajei [Ophiuroidea] considered in relation to their distribution. J. Mar. Biol. Assoc. UK 44, 565–576. ( 10.1017/S0025315400027776) [DOI] [Google Scholar]
- 64.Zuo X, Yue X, Lv P, Yu Q, Chen M, Zhang J, Luo Y, Wang S, Zhang J. 2017. Contrasting effects of plant inter-and intraspecific variation on community trait responses to restoration of a sandy grassland ecosystem. Ecol. Evol. 7, 1125–1134. ( 10.1002/ece3.271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matesanz S, Ramírez-Valiente JA. 2019. A review and meta-analysis of intraspecific differences in phenotypic plasticity: implications to forecast plant responses to climate change. Glob. Ecol. Biogeogr. 28, 1682–1694. ( 10.1111/geb.12972) [DOI] [Google Scholar]
- 66.Henn JJ, et al. 2018. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 9, 1548 ( 10.3389/fpls.2018.01548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mensens C, De Laender F, Janssen CR, Sabbe K, De Troch M. 2017. Different response–effect trait relationships underlie contrasting responses to two chemical stressors. J. Ecol. 105, 1598–1609. ( 10.1111/1365-2745.12777) [DOI] [Google Scholar]
- 68.Derroire G, Powers JS, Hulshof CM, Varela LEC, Healey JR. 2018. Contrasting patterns of leaf trait variation among and within species during tropical dry forest succession in Costa Rica. Sci. Rep. 8, 285 ( 10.1038/s41598-017-18525-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright JP, Ames GM, Mitchell RM. 2016. The more things change, the more they stay the same? When is trait variability important for stability of ecosystem function in a changing environment. Phil. Trans. R. Soc. B 371, 20150272 ( 10.1098/rstb.2015.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cassidy C, Grange LJ, Garcia C, Bolam SG, Godbold JA. 2020. Data from: Species interactions and environmental context affect intraspecific behavioural trait variation and ecosystem function Dryad Digital Repository. ( 10.5061/dryad.14t4h37) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cassidy C, Grange LJ, Garcia C, Bolam SG, Godbold JA. 2020. Data from: Species interactions and environmental context affect intraspecific behavioural trait variation and ecosystem function Dryad Digital Repository. ( 10.5061/dryad.14t4h37) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Supporting information for this article have been uploaded as the electronic supplementary material. Raw data are archived at the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.14t4h37 [70].