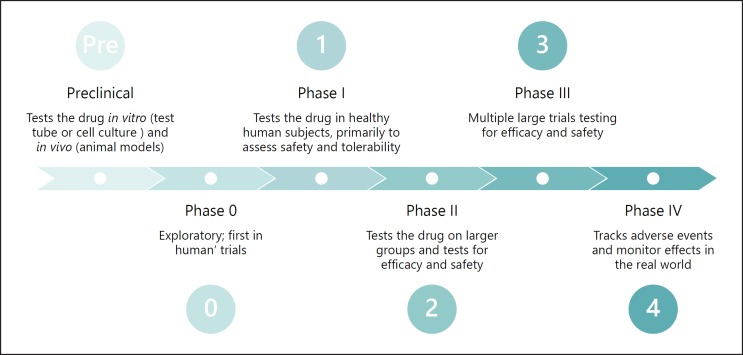
Fig. 3.
The phases of clinical trial research. Clinical trials pass through a series of phases as the trial sponsor gains more evidence around the investigational drug or biologic. Preclinical studies are often conducted in cell and animal models (e.g., on mice), and then are slowly expanded into humans. First in healthy humans in small numbers to test safety, and then to larger sets of humans who have the condition in question to test both safety and efficacy.