Abstract
Background:
Inflammatory effects of ambient particulate matter (PM) air pollution exposures may underlie PM-related increases in cardiovascular disease risk and mortality, although evidence of PM-associated leukocytosis is inconsistent and largely based on small, cross-sectional, and/or unrepresentative study populations.
Objectives:
Our objective was to estimate PM–leukocyte associations among U.S. women and men in the Women’s Health Initiative and Atherosclerosis Risk in Communities study ().
Methods:
We based the PM–leukocyte estimations on up to four study visits per participant, at which peripheral blood leukocytes and geocoded address-specific concentrations of , , and in diameter (, , and , respectively) were available. We multiply imputed missing data using chained equations and estimated PM–leukocyte count associations over daily to yearly PM exposure averaging periods using center-specific, linear, mixed, longitudinal models weighted for attrition and adjusted for sociodemographic, behavioral, meteorological, and geographic covariates. In a subset of participants with available data (), we also estimated PM–leukocyte proportion associations in compositional data analyses.
Results:
We found a (95% confidence interval: , 33) higher leukocyte count, a 1.2% (0.6%, 1.8%) higher granulocyte proportion, and a (, ) lower T-cell proportion per increase in 1-month mean . However, shorter-duration exposures were inversely and only modestly associated with leukocyte count.
Discussion:
The –leukocyte estimates, albeit imprecise, suggest that among racially, ethnically, and environmentally diverse U.S. populations, sustained, ambient exposure to fine PM may induce subclinical, but epidemiologically important, inflammatory effects. https://doi.org/10.1289/EHP5360
Introduction
Exposures to airborne particulate matter (PM) , and between 2.5 and in diameter (, , and , respectively) can trigger inflammatory responses that involve the release and hematogenous redistribution of leukocytes (Pope et al. 2016; Tan et al. 2000; Terashima et al. 1997a). Such responses may be key to the pathophysiology underpinning established associations between ambient PM, cardiovascular (CVD) disease risk, and mortality (Brook et al. 2010; Chi et al. 2016a; Di et al. 2017; Miller et al. 2007; Parker et al. 2018). However, evidence of PM-associated leukocytosis is inconsistent and mostly based on small studies and panels with limited generalizability (Brook et al. 2009; Dubowsky et al. 2006; Emmerechts et al. 2012; Ghio et al. 2003; Gong et al. 2004; Huang et al. 2014; Jacobs et al. 2010; Mills et al. 2005, 2007; Pope et al. 2004, 2016; Riediker 2007; Salvi et al. 1999; Steenhof et al. 2014; Törnqvist et al. 2007).
In larger, community- and population-based studies, short-duration –leukocyte count associations are similarly inconsistent (Liao et al. 2005; Schwartz 2001; Seaton et al. 1999; Steinvil et al. 2008), although longer-duration – and –leukocyte count associations tend to be positive in published cross-sectional and longitudinal studies (Chen and Schwartz 2008; Chuang et al. 2011; Viehmann et al. 2015). Moreover, associations between short- and longer-term PM exposures and leukocyte count and its differential composition have not been thoroughly evaluated while controlling for known relationships among leukocyte traits (count and component proportions).
Associations between ambient PM exposures and leukocyte traits could nevertheless lend support to the hypothesized role of inflammation in PM-related pathogenesis. Furthermore, their magnitude would provide insight into PM associations with leukocyte-derived biomarkers such as DNA methylation (DNAm), a heritable but dynamic epigenetic modification that can influence gene expression. Indeed, epidemiologic studies often rely on peripheral blood leukocytes as a source of DNA for DNAm assays given the relative ease with which they are collected and archived in large populations (McCullough et al. 2017; Zhong et al. 2016). Because DNAm and other epigenetic biomarkers (Beaulieu et al. 2017) differ among leukocyte subtypes [e.g., granulocytes vs. monocytes (Houseman et al. 2012; Jaffe and Irizarry 2014)], leukocyte composition may plausibly mediate their associations with environmental exposures.
To expand on prior work evaluating PM–leukocyte count associations, and to address the limitations of studies examining PM–leukocyte compositional associations, we estimated associations of leukocyte traits with short- to longer-duration exposures to ambient , , and in large, multiracial/ethnic, and geographically diverse United States populations enrolled in the Women’s Health Initiative (WHI) and the Atherosclerosis Risk in Communities (ARIC) study.
Methods
Study Populations
The WHI is a multicenter prospective study of risk factors for CVD, breast/colorectal cancer, and osteoporotic fractures (Women’s Health Initiative Study Group 1998, Anderson et al. 2003). From forty clinical centers throughout the United States, postmenopausal women aged 50–79 years of age were either randomized in the Clinical Trials (CT; ) or enrolled in the Observational Study (OS; ) between 1993 and 1998. The WHI CT included three interventions: a) hormone therapy (i.e., estrogen with or without progestin vs. placebo), b) calcium and vitamin D supplementation (vs. placebo), and c) dietary modification (vs. usual diet). The WHI OS (Women’s Health Initiative Study Group 1998, Anderson et al. 2003) recruited participants interested in the dietary modification or hormone therapy trials of the WHI CT but were otherwise ineligible, unwilling, or unresponsive to a direct invitation.
The WHI CT and OS participants completed a baseline screening visit, at which fasting blood and other demographic, socioeconomic, behavioral, and medical information was collected by trained and certified staff. The present study additionally included WHI CT participant data from triennial follow-up visits 3 and 6 y after randomization (Annual Visits 3 and 6) and WHI OS participant data 3 y after enrollment (Annual Visit 3), at which fasting blood was redrawn.
The ARIC study is a prospective epidemiologic study of atherosclerosis and CVD in four U.S. communities: Washington County, Maryland; Forsyth County, North Carolina; selected suburbs of Minneapolis, Minnesota; and Jackson, Mississippi (ARIC Investigators 1989). Participants were selected as a community-stratified probability sample of 15,792 mostly African- and European-American men and women 45–64 years of age and participated in a baseline exam (Visit 1; 1987–1989) at which fasting blood and other demographic, socioeconomic, behavioral, and medical information was collected by trained and certified staff. The present study also included participant data from up to three triennial follow-up visits 3, 6, and 9 y after enrollment (Visits 2–4, 1990–1998) during which fasting blood was redrawn.
Leukocyte composition analyses were conducted in five WHI and ARIC subpopulations with available DNAm data (see Table S1). The three WHI subpopulations included a) Ancillary Study 315 (WHI-EMPC; ) (Whitsel 2018), b) Broad Agency Announcement 23 (WHI-BAA23; ) (Assimes et al. 2018), and c) Ancillary Study 311 (WHI-AS311; ) (Bhatti 2018). WHI-EMPC, also known as Epigenetic Mechanisms of PM-Mediated CVD Risk, is a study of epigenetic mechanisms underlying associations between PM and CVD within randomly selected WHI CT participants at the screening visit, Annual Visit 3, or Annual Visit 6. WHI-BAA23, also known as Integrative Genomics and Risk of CHD and Related Phenotypes in the Women’s Health Initiative, is a case–control study of coronary heart disease. By design, WHI-BAA23 oversampled African Americans and Hispanic/Latino Americans and required all participants to have undergone genome-wide genotyping and profiling of seven CVD biomarkers. DNAm was measured in blood collected at the screening visit, before the incidence of coronary heart disease. WHI-AS311, also known as the Bladder Cancer and Leukocyte Methylation study, is a nested case–control study of bladder cancer. Bladder cancer cases were matched to controls based on enrollment year, age at enrollment, follow-up time, and DNAm extraction method. DNAm was measured in blood collected at the screening visit, before the incidence of bladder cancer. The two ARIC subpopulations included 2,796 African Americans from Forsyth County or Jackson (ARIC-AA) with DNA and 1,139 European Americans from Forsyth County, Minneapolis, or Washington County (ARIC-EA) with cerebral magnetic resonance imaging data (Mosley et al. 2005) all at Visits 2 (1990–1992) or 3 (1993–1995) (see Figure S1).
Leukocyte Counts and Composition
Leukocyte counts were measured among WHI CT participants at the screening visit, among OS participants at the screening visit and Annual Visit 3, and among ARIC participants at Visits 1–2 on automated cell counters at local laboratories following standard quality assurance procedures (Papp et al. 1989). Leukocyte counts were remeasured among ARIC participants in Washington County at Visits 3–4 and in Forsyth County at Visit 4. Table 1 displays the number of included participants with leukocyte count data, by study and visit. Established associations between leukocyte count, demographic, and clinical variables in WHI and ARIC have been reported by others (Margolis et al. 2005; Nieto et al. 1992).
Table 1.
Characteristics of participants with leukocyte count data before imputation, Women’s Health Initiative (1993–2002) and Atherosclerosis Risk in Communities (1986–1998) study.
| Characteristic | WHI screening visit and ARIC visit 1 | WHI | ARIC | WHI and ARIC | ||||
|---|---|---|---|---|---|---|---|---|
| Screening visit | Annual visit 3a | Visit 1 | Visit 2 | Visit 3b | Visit 4c | Percentage imputed of 285,548 observations (%) | ||
| Male [n (%)] | 6,563 (4.1) | 0 (0.0) | 0 (0.0) | 6,563 (45.5) | 5,892 (45.3) | 1,470 (47.4) | 2,497 (46.0) | 0 |
| Age [y ()] | 0 | |||||||
| Race/ethnicity [n (%)] | 0.2 | |||||||
| American Indian or Alaskan Native | 658 (0.4) | 647 (0.4) | 315 (0.4) | 11 (0.1) | 10 (0.1) | 2 (0.1) | 5 (0.1) | |
| Asian or Pacific islander | 1,633 (1.0) | 1,601 (1.1) | 1,018 (1.3) | 32 (0.2) | 29 (0.2) | 9 (0.3) | 16 (0.3) | |
| Black or African American | 15,809 (10.0) | 11,990 (8.3) | 5,675 (7.4) | 3,819 (26.5) | 3,221 (24.8) | 25 (0.8) | 244 (4.5) | |
| Hispanic/Latino | 5,967 (3.8) | 5,967 (4.1) | 2,681 (3.5) | —d | —d | —d | —d | |
| Other | 1,353 (0.9) | 1,353 (0.9) | 740 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| White (not of Hispanic origin) or European American | 133,400 (84.0) | 122,844 (85.1) | 66,457 (86.4) | 10,556 (73.2) | 9,740 (74.9) | 3,064 (98.8) | 5,168 (95.1) | |
| Education [n (%)] | 0.6 | |||||||
| High school education or lower | 40,473 (25.6) | 32,358 (22.5) | 15,677 (20.5) | 8,115 (56.4) | 7,136 (55.0) | 2,115 (68.3) | 3,148 (58.0) | |
| More than high school | 117,654 (74.4) | 111,377 (77.5) | 60,818 (79.5) | 6,277 (43.6) | 5,842 (45.0) | 982 (31.7) | 2,279 (42.0) | |
| Smoking status [n (%)] | 2.0 | |||||||
| Never | 78,794 (50.1) | 72,760 (50.9) | 37,749 (51.1) | 6,034 (41.9) | 5,173 (39.9) | 1,378 (44.5) | 2,270 (41.9) | |
| Former | 64,941 (41.2) | 60,314 (42.2) | 32,708 (44.2) | 4,627 (32.1) | 4,897 (37.7) | 1,259 (40.6) | 2,331 (43.0) | |
| Current | 13,564 (8.6) | 9,821 (6.9) | 3,465 (4.7) | 3,743 (26.0) | 2,909 (22.4) | 463 (14.9) | 822 (15.2) | |
| Alcohol use [n (%)] | 1.4 | |||||||
| Never | 18,683 (11.8) | 15,101 (10.5) | 6,807 (9.1) | 3,582 (24.9) | 2,917 (22.5) | 783 (25.3) | 1,273 (23.5) | |
| Former | 28,972 (18.3) | 26,274 (18.3) | 15,040 (20.1) | 2,698 (18.8) | 2,678 (20.6) | 761 (24.6) | 1,680 (31.0) | |
| Current | 110,366 (69.8) | 102,289 (71.2) | 52,866 (70.8) | 8,077 (56.3) | 7,384 (56.9) | 1,554 (50.2) | 2,474 (45.6) | |
| Body mass index [ ()] | 3.2 | |||||||
| Physical activity [MET-h/week ()] | 3.6 | |||||||
| Neighborhood SES (z-score sum) | 0.2 (5.3) | 0.0 (5.4) | (5.4) | (2.9) | 0.4 (4.4) | 9.1 | ||
| Leukocyte count [ ()] | 3.9 | |||||||
Note: ARIC, Atherosclerosis Risk in Communities; SD, standard deviation; SES, socioeconomic status; WHI, Women’s Health Initiative.
WHI Observational Study participants only.
Participants from Washington County only.
Participants from Forsyth County (46%) or Washington County (54%).
ARIC recruitment and data collection occurred before the National Institutes of Health required collection of information about Hispanic/Latino ethnicity.
Leukocyte composition [i.e., the proportions of T cells, T cells, natural killer (NK) cells, B cells, monocytes, and granulocytes] were validly estimated (Houseman et al. 2012) among a subset of WHI and ARIC participants with DNAm data using methods that leverage differentially methylated regions [i.e., stably methylated CpG sites within, but variably methylated CpG sites among leukocyte cell types (Houseman et al. 2012; Koestler et al. 2013)]. Table S2 displays the number of included participants with leukocyte composition data, by subpopulation.
Particulate Matter Exposure Estimation
The study focused on , , and (coarse) , the first two of which are regulated under the Clean Air Act by the U.S. Environmental Protection Agency (EPA) (U.S. EPA 2017). PM exposures were based on either daily or monthly estimation methods. Daily mean concentrations (in micrograms per cubic meter) of were spatially estimated at all geocoded participant addresses (Whitsel et al. 2004, 2006) using U.S. EPA Air Quality System (AQS) data and national-scale, log-normal ordinary kriging (Liao et al. 2006, 2007). For each participant, daily mean concentrations of were averaged over 2 and 7 d prior to and including the day of the study visit.
Geocoded participant address-specific monthly mean concentrations (in micrograms per cubic meter) of and were spatiotemporally estimated using generalized additive mixed models and geographic information system–based predictors. Because U.S. EPA AQS monitoring data for were not widely available until 1999, spatiotemporal estimation also involved the log-transformed ratio of to predicted between 1987 and 1999 (Yanosky et al. 2014). Monthly mean concentrations were averaged over the 12 months prior to and including examination months to obtain annual means. concentrations for 1- and 12-month means were defined as the monthly differences between and concentrations.
Covariates
Demographic, socioeconomic, behavioral, and medical covariates included study center, visit, self-identified race/ethnicity, age (in years), individual-level education (high school education or lower, more than high school), neighborhood socioeconomic status (Diez Roux et al. 2001), smoking status (current, former, never), alcohol use (current, former, never), measured body mass index (BMI; in kilograms per squared meters), total energy expenditure [metabolic equivalent of task (MET)-hours/week], mean temperature (in degrees Celsius), mean dew point (in degrees Celsius), mean barometric pressure (in kilopascals), season (using sine/cosine functions) (Stolwijk et al. 1999), and to control for longer-term temporal trends, an interval-scale variable for calendar date. Race/ethnicity and individual-level education were self-reported at baseline. Smoking status, alcohol use, BMI, and total energy expenditure were evaluated at each study visit, the latter based on the type, frequency, and duration of recreational physical activity (Manson et al. 2002). When physical activity information was unavailable, it was defined as the value given at the last visit or the weighted mean between visits if data were available. Geocoded participant address-specific neighborhood socioeconomic status was a sum of Z-transformed U.S. Census tract-level measures of median household income; percent of households with interest dividends or rent income; percent of population at least 25 years of age with a high school degree; percent of population at least 16 years of age with professional, managerial, or executive occupations; and median value of owner-occupied housing units (Diez Roux et al. 2001). Geocoded participant address-specific daily mean temperature, dew point, and barometric pressure were averaged across all National Climatic Data Center monitoring stations within (NCDC 2019), then averaged over 2, 7, 28, and 365 d prior to and including the day of the study visit.
Subpopulation-specific covariates included sex (in ARIC), randomly assigned treatment group (in WHI), case–control status (in WHI-AS311 and WHI-BAA23), and other sampling-related variables in WHI-AS311 (i.e., enrollment year, age at enrollment, follow-up time, DNAm extraction method).
Exclusions
Of all observations in WHI and ARIC (), small percentages were excluded because they were made on participants in one WHI center outside of the contiguous 48 states (2%), on study visit dates for which PM was not estimable (2%), among participants with a study-specific leukocyte (leukocytosis, 0.5%), study-specific leukocyte (leukopenia, 0.5%), or conditions associated with abnormal leukocyte traits such as hematological malignancy (1.7%) or oral/parenteral use of a granulocyte/macrophage colony stimulating factor (), lithium (0.2%), glucocorticosteroid (1.1%), or antibiotic use (2.6%).
Multiple Imputation
To avoid potential for selection bias in complete-data analyses when data are missing at random (Hernán et al. 2004), multivariate imputation by chained equations (MICE) (Azur et al. 2011; Stuart et al. 2009) was used to impute missing data (percentage missing range: 0.6–9.1%). Binary and categorical data were imputed using logistic regression, and continuous variables were imputed using predictive means matching.
Attrition Weights
To address the potential for bias due to nonrandom attrition over time in leukocyte count analyses in WHI and ARIC, stabilized inverse probability weights for each participant were calculated at each examination using logistic regression, where the numerator was the marginal probability of the participant not being lost to follow-up at an examination and the denominator was the probability of the participant not being lost to follow-up at an examination conditional on their covariate patterns at prior examination (Howe et al. 2016).
Statistical Analysis: Leukocyte Count
Study- and center-stratified, PM–leukocyte count associations were estimated using an attrition-weighted and covariate-adjusted, two-level, linear, mixed-effects, longitudinal model including a random intercept for examination at the participant level. The model was given by
| [1] |
where i and j denote the ith examination (level 1) of the jth participant (level 2); LC is the leukocyte count; is the intercept; PM is the 2- or 7-d mean of or the 1- or 12-month mean of , , or ; and Z is a vector of covariates. The term is a random intercept for examination at the participant level to account for within-participant variation, and is the random error at the examination level. Study- and center-specific measures of association () and their 95% confidence intervals (CIs) were estimated as per increase in PM, forest plotted, and pooled in random-effects meta-analyses (DerSimonian and Laird 1986) after testing homogeneity of associations among strata () (Cochran 1954).
Statistical Analysis: Leukocyte Composition
Subpopulation-stratified, cross-sectional, PM–leukocyte proportion associations were analyzed using multivariate methods for compositional data (Aitchison 1982; Egozcue et al. 2003), that is, a set of positive, mutually exclusive components (such as proportions, p) that represent parts constituting a whole, are multicollinear, and collectively sum to 1 within a constrained space called a simplex. Proportions were isometrically log-ratio (ilr)-transformed from the simplex to real (Euclidean geometric) space. Transformation—which allowed for the dependent variation (Chastin et al. 2015; Egozcue et al. 2003) and relative positioning of components in the simplex (Chastin et al. 2015; Fairclough et al. 2017)—resulted in p-1 orthogonal (i.e., non-multicollinear) coordinates. It also allowed for back-transformation of multivariate results into component proportions (Pawlowsky-Glahn et al. 2015). Back-transformation was based on compositional data analysis models, as given by
| [2] |
where denotes the ilr-transformed estimated leukocyte proportions; is the intercept; PM is the 2- or 7-d mean of or the 1- or 12-month mean of , , or ; Z is a vector of covariates; and is the random error term. The vector of association measures () denotes the five orthogonal coordinates, the back-transformation of which represents the corresponding difference in each of the six leukocyte proportions per increase in PM. Because the SEs of cannot be back-transformed, the SEs of back-transformed leukocyte proportion associations were estimated using 1,000 bootstrap samples. Subpopulation-specific measures of association were reported as absolute percentage differences (%), forest plotted, and pooled in random effects meta-analyses (DerSimonian and Laird 1986) after testing homogeneity of associations among strata () (Cochran 1954).
Statistical Analysis: Sensitivity
In leukocyte count analyses, Model 1 adjusted for self-identified race/ethnicity, age, sex (in ARIC), randomly assigned treatment group (in WHI), visit, mean temperature, mean dew point, mean barometric pressure, season (to control for within-year variation), and a restricted cubic natural spline function of calendar date (Bhaskaran et al. 2013; Dominici et al. 2002; Peng et al. 2006) with one knot per year to control for secular trends in PM and leukocyte count methods. Model 2 also adjusted for potential socioeconomic confounders (individual-level education and neighborhood socioeconomic status). Model 3 additionally adjusted for behavioral variables that explain variation in leukocyte traits or account for residual confounding (smoking status, alcohol use, BMI, and physical activity). The sensitivity of Model 3 results to the use of two knots per calendar year, one knot for every two calendar years, and no calendar date adjustment was assessed. Although leukocyte composition analyses also adjusted for subpopulation-specific covariates, the models did not adjust for calendar date because leukocyte proportions were estimated using the same methods across subpopulations. In addition, leukocyte composition models were not center-stratified due to small sample sizes and instead were adjusted for U.S. Census region (Midwest, Northeast, South, and West). Sensitivity of leukocyte count associations to PM estimation method was examined by substituting spatially estimated 28- and 365-d mean concentrations of for spatiotemporally estimated 1- and 12-month mean concentrations of . Sensitivity of significant PM-estimated leukocyte composition associations were assessed in a subset of ARIC participants with available measured leukocyte composition data (lymphocyte, monocyte, and granulocyte proportions). Additional sensitivity of PM–leukocyte composition associations were evaluated by estimating PM associations with the log-transformed ratio of to T-cell proportions (CD4:CD8)—a marker of immune function and possible biomarker for coronary heart disease (Neupane et al. 2019). PM–CD4:CD8 associations were reported as percentage changes.
Results
Of the 150,328 WHI participants and 15,347 ARIC participants with leukocyte count data (total ; Figure 1), 96% and 94% had baseline data after exclusions. At baseline, participants were 62.3 years of age on average and mostly female (96%), white (84%), more than high school educated (74%), never/former smokers (91%), and current alcohol users (70%). Mean BMI, physical activity, and leukocyte count were , 12.3 MET-hours/week, and (Table 1). Participants in the WHI and ARIC subpopulations with leukocyte composition data (; Table S2) were more likely to be younger (mean age: 61.5 y) and male (16%) and less likely to be white (45%), more than high school educated (52%), never/former smokers (85%), and current alcohol users (52%) than those with leukocyte count data. Among these subpopulations, mean estimated leukocyte cell type percentages were 9% ( T cells), 18% ( T cells), 7% (natural killer cells), 7% (B cells), 10% (monocytes), and 49% (granulocytes).
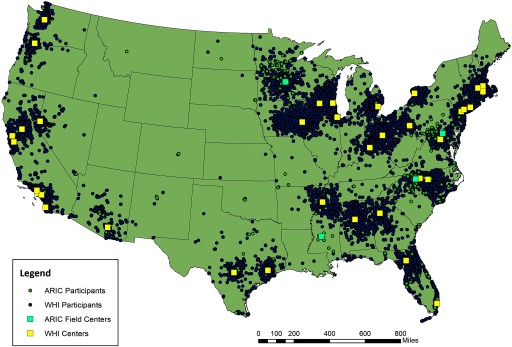
Figure 1.
Map of geocoded Women’s Health Initiative (1993–2002) and Atherosclerosis Risk in Communities (1986–1998) study participants and centers at baseline. WHI centers () followed 1,238–3,690 participants. ARIC centers followed 3,588–3,943 participants. WHI and ARIC centers were co-located in Minneapolis, MN, and Winston-Salem, NC.
Mean concentrations in the populations with leukocyte count and composition data were below U.S. EPA National Ambient Air Quality Standards (NAAQS) in place during the study period (24-h ; annual ) (U.S. EPA 2017). However, 1- and 12-month mean concentrations in ARIC approached or exceeded the annual standard in place during the study period () (Table 2; Table S3). and concentrations were higher, whereas concentrations were lower among subpopulations with leukocyte composition data.
Table 2.
Particulate matter concentrations among participants with leukocyte count data before imputation, Women’s Health Initiative (1993–2002) and Atherosclerosis Risk in Communities (1986–1998) study.
| PM () | WHI screening visit and ARIC visit 1 | WHI | ARIC | WHI and ARIC | ||||
|---|---|---|---|---|---|---|---|---|
| Screening visit | Annual visit 3a | Visit 1 | Visit 2 | Visit 3b | Visit 4c | Percentage imputed of 285,548 observations (%) | ||
| 2-d | 5.5 | |||||||
| 7-d | 5.5 | |||||||
| 1-month | 7.0 | |||||||
| 12-month | 8.9 | |||||||
| 1-month | 7.0 | |||||||
| 12-month | 8.9 | |||||||
| 1-month | 7.0 | |||||||
| 12-month | 8.9 | |||||||
Note: ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; PM, particulate matter; , in diameter; , in diameter; , and in diameter; WHI, Women’s Health Initiative.
WHI Observational Study participants only.
Participants from Washington County only.
Participants from Forsyth County (46%) or Washington County (54%).
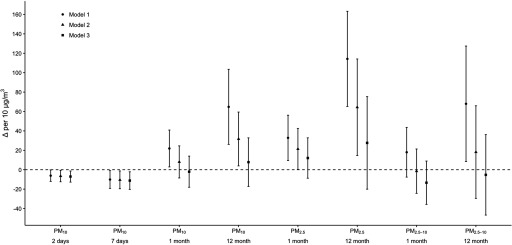
In Models 1–3, short-term mean concentrations were inversely associated with leukocyte count when pooled across study- and center-specific strata. For example, in Model 3, there were 7 (95% CI: , ) and 11 (, ) lower leukocyte counts per increase in 2- and 7-d mean concentration (Table 3; Figure 2).
Table 3.
Pooled difference in leukocyte count (; ) per increase in PM concentrations among participants, Women’s Health Initiative (1993–2002) and Atherosclerosis Risk in Communities (1986–1998) study.
| PM exposure | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| (95% CI) | d | (95% CI) | d | (95% CI) | d | |
| () | ||||||
| 2-d mean | (, 0) | 0.89 | (, ) | 0.90 | (, ) | 0.91 |
| 7-d mean | (, ) | 0.49 | (, ) | 0.53 | (, ) | 0.42 |
| 1-month mean | 22 ( 3, 41) | 2.5 | 8 (, 25) | 0.08 | (, 14) | 0.08 |
| 12-month mean | 65 (26, 103) | 6.5 | 32 (4, 59) | 0.37 | 8 (, 33) | 0.56 |
| () | ||||||
| 1-month mean | 33 (9, 56) | 0.21 | 21 (0, 43) | 0.51 | 12 (, 33) | 0.45 |
| 12-month mean | 114 (65, 163) | 0.59 | 64 (15, 114) | 0.99 | 28 (, 75) | 0.99 |
| () | ||||||
| 1-month mean | 18 (, 44) | 0.01 | (, 21) | 0.13 | (, 9) | 0.12 |
| 12-month mean | 67 (8, 127) | 6.5 | 18 (, 66) | 0.04 | (, 36) | 0.15 |
Note: ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; PM, particulate matter; , in diameter; , in diameter; , and in diameter; WHI, Women’s Health Initiative.
Model 1 adjusted for race/ethnicity, age, sex (in ARIC), randomly assigned treatment group (in WHI), mean temperature, mean dew point, mean barometric pressure, season, and a restricted cubic natural spline function of calendar time with one knot per calendar year.
Model 2 adjusted for all covariates in Model 1 and additionally for individual-level education and neighborhood socioeconomic status.
Model 3 adjusted for all covariates in Model 2 and additionally for smoking status, alcohol use, body mass index, and physical activity.
Homogeneity of associations among strata was tested using Cochran’s Q-test statistic, where a suggests there is evidence to reject the null hypothesis of homogeneity.
Figure 2.
Pooled difference in leukocyte count (; ) per increase in PM concentrations among participants, Women’s Health Initiative (1993–2002) and Atherosclerosis Risk in Communities (1986–1998) study. Model 1 adjusted for race/ethnicity, age, sex (in ARIC), randomly assigned treatment group (in WHI), mean temperature, mean dew point, mean barometric pressure, season, and a restricted cubic natural spline function of calendar date with one knot per year. Model 2 adjusted for all covariates in Model 1 plus individual-level education and neighborhood socioeconomic status. Model 3 adjusted for all covariates in Model 2 plus smoking status, alcohol use, body mass index, and physical activity.
In Model 1, longer-term mean , , and concentrations were positively, but imprecisely, associated with the leukocyte count (i.e., they had wide CIs). However, the associations also were attenuated by additional adjustment for potential socioeconomic confounders (Model 2) and behavioral variables (Model 3). For example, there were 114 (65, 163), 64 (15, 114), and 28 (, 75) higher leukocyte counts per increase in the 12-month mean concentration in Models 1–3 (Table 3; Figure S2). In sensitivity analyses, estimates were generally robust to variation in the method of controlling for calendar date (see Figure S3). Leukocyte count associations with 28- and 365-d mean concentrations also were imprecise and no different from the null associations (see Table S4), as those between leukocyte count and 1- and 12-month mean .
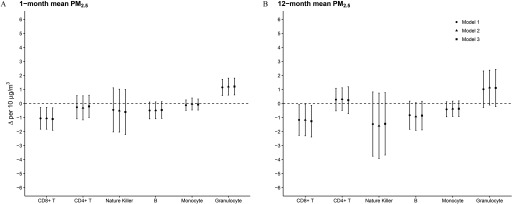
Across PM size fractions and averaging durations, PM–leukocyte compositional associations in Model 3 (Table 4) differed little from those in Models 1 and 2 (see Tables S5–S6). Higher 7-d mean concentrations were associated with somewhat higher T-cell proportions, whereas 1- and 12-month mean concentrations were associated with somewhat lower T-cell proportions (Table 4; Figure S4). One- and 12-month mean concentrations of were associated with lower T-cell, NK cell, and B-cell proportions and higher granulocyte proportions. For example, there was a 1.1% (, ) lower T-cell proportion and 1.2% (0.6%, 1.8%) higher granulocyte proportion per increase in 1-month mean (Figure 3). In contrast, there were 0.6% (, 0.1%) and 1.2% (, 0.1%) lower granulocyte proportions per 1- and 12-month mean (see Figure S4). associations with estimated granulocyte proportions were consistent in magnitude and direction with those in the analyses of measured granulocyte proportions (see Table S7). PM–CD4:CD8 associations were generally inconsistent, with suggestively inverse associations with short-duration and suggestively positive associations with longer duration and ; however, CIs were wide and included the null (Table S8).
Table 4.
Pooled difference in estimated leukocyte proportion (; %) per increase in PM concentrations among participants, Women’s Health Initiative (1993–2002) and Atherosclerosis Risk in Communities (1990–1995) study.
| PM exposure | T cells | T cells | Natural killer cells | B cells | Monocytes | Granulocytes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI)a | b | % (95% CI)a | b | % (95% CI)a | b | % (95% CI)a | b | % (95% CI)a | b | % (95% CI)a | b | |
| () | ||||||||||||
| 2-d mean | 0.1 (, 0.6) | 0.15 | (, 0.2) | 0.12 | 0.0 (, 0.3) | 0.93 | (, 0.0) | 0.46 | 0.0 (, 0.2) | 0.49 | 0.1 (, 0.3) | 0.69 |
| 7-d mean | 0.3 (, 0.8) | 0.28 | (, 0.1) | 0.63 | (, 0.4) | 0.18 | (, ) | 0.93 | (, 0.2) | 0.49 | (, 0.2) | 0.25 |
| 1-month mean | (, 0.3) | 0.30 | 0.0 (, 0.5) | 0.26 | (, 0.4) | 0.16 | (, 0.2) | 0.64 | 0.2 (, 0.8) | 0.06 | 0.4 (, 0.9) | 0.28 |
| 12-month mean | (, 0.4) | 0.63 | 0.1 (, 0.6) | 0.38 | (, 0.8) | 0.14 | (, 0.2) | 0.58 | (, 0.1) | 0.41 | 0.0 (, 1.0) | 0.13 |
| () | ||||||||||||
| 1-month mean | (, ) | 0.58 | (, 0.6) | 0.18 | (, 1.0) | 0.00 | (, 0.1) | 0.72 | (, 0.3) | 0.44 | 1.2 (0.6, 1.8) | 0.75 |
| 12-month mean | (, ) | 0.84 | 0.2 (, 1.2) | 0.34 | (, 0.8) | 0.03 | (, 0.2) | 0.42 | (, 0.2) | 0.65 | 1.1 (, 2.4) | 0.25 |
| () | ||||||||||||
| 1-month mean | 0.5 (, 1.8) | 0.23 | 0.0 (, 0.6) | 0.80 | (, 0.7) | 0.63 | (, 0.5) | 0.80 | 0.1 (, 0.8) | 0.14 | (, 0.1) | 0.33 |
| 12-month mean | 0.0 (, 2.3) | 0.13 | (, 0.6) | 0.51 | 0.3 (, 1.4) | 0.80 | (, 0.7) | 0.71 | (, 0.4) | 0.60 | (, 0.1) | 0.17 |
Note: ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; PM, particulate matter; , in diameter; , in diameter; , and in diameter; WHI, Women’s Health Initiative.
Model adjusted for race/ethnicity, age, sex (in ARIC), randomly assigned treatment group (in WHI), mean temperature, mean dew point, mean barometric pressure, season, individual-level education, neighborhood socioeconomic status, smoking status, alcohol use, body mass index and physical activity.
Homogeneity of associations among strata was tested using the Cochran’s Q-test statistic, where a suggests there is evidence to reject the null hypothesis of homogeneity.
Figure 3.
Pooled difference in leukocyte composition (; %) per increase in (A) 1- and (B) 12-month mean concentrations among participants, Women’s Health Initiative (1993–2002) and Atherosclerosis Risk in Communities (1990–1995) study. Model 1 adjusted for race/ethnicity, age, sex (in ARIC), randomly assigned treatment group (in WHI), mean temperature, mean dew point, mean barometric pressure, season, and subpopulation-specific covariates. Model 2 also adjusted for individual-level education and neighborhood socioeconomic status. Model 3 additionally adjusted for smoking status, alcohol use, body mass index, and physical activity.
Discussion
Results from this study suggest that mid- to longer-duration exposures to concentrations below U.S. EPA NAAQS may be associated with a higher leukocyte count, higher granulocyte proportion, and lower T-cell proportion among multi-ethnic and geographically diverse populations of U.S. women and men.
Although leukocyte count associations were also observed with 1- and 12-month mean and concentrations, adjusting for potential socioeconomic confounders attenuated them. Indeed, lower socioeconomic status has been related both to increases in CVD risk (Elo 2009) and higher concentrations of ambient PM (Hajat et al. 2015). Further attenuation was observed with additional adjustment for behavioral variables (smoking, alcohol use, BMI, and physical activity) suggesting that they may account for residual confounding by socioeconomic or other unmeasured characteristics. Taken together with prior evidence suggesting positive (Chen and Schwartz 2008) and null (Viehmann et al. 2015) associations between longer-duration with leukocyte counts, the present results were unable to clarify the relationship. Nevertheless, positive—yet imprecise—leukocyte count estimates remained for , supporting evidence first reported in the Heinz Nixdorf Recall study (Viehmann et al. 2015). Moreover, the magnitudes of estimates presently observed are on par with those previously associated with a 1-cigarette/d increase in smoking (Hansen et al. 1990; Petitti and Kipp 1986; Schwartz and Weiss 1991).
concentrations were also associated with leukocyte composition; particularly, with higher granulocyte and lower T-cell proportions. This observation is consistent with results from the Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan that found positive associations between long-duration exposure and the proportion of neutrophils, the most abundant type of granulocyte (Chuang et al. 2011). SEBAS also detected similar associations with long-duration concentrations, but they were not observed in the present study. Results are also consistent with small-scale occupational studies that found higher neutrophil (Riediker et al. 2004) and lower lymphocyte/ T-cell proportions (Riediker et al. 2004; Zhao et al. 2013) albeit with short-duration exposure to , which was further demonstrated in rats (Gerlofs-Nijland et al. 2005; Gordon et al. 1998; Kodavanti et al. 2002). Indeed, observed lower T-cell proportions may be related to PM-responsive migration of lymphocytes from the blood to bronchial tissues (Salvi et al. 1999), contraction of the regulatory (suppressor) T-cell pool, and/or latter phase homeostatic contraction of the cytotoxic T-cell pool (Huang et al. 1999).
Persistent systemic inflammation due to longer-duration PM exposure is a biologically plausible mechanism linking PM with adverse health. Indeed, systemic inflammation has been implicated in endothelial injury, atherosclerotic disease progression, and subsequent increases in CVD risk (Ross 1999). In the epidemiologic context, systemic inflammation, as measured by leukocyte count, has been consistently and independently associated with CVD and mortality in WHI (Kabat et al. 2017; Margolis et al. 2005), in ARIC (Lee et al. 2001), and in other populations (Brown et al. 2001; Danesh et al. 1998; Ruggiero et al. 2007).
The results presented herein support the hypothesis that chronic exposure to PM contributes to systemic inflammation and may partly explain the established connection between PM and CVD risk (Chi et al. 2016a; Miller et al. 2007). They support prior studies that mechanistically linked atherosclerosis and the inflammatory responses to PM (Adar et al. 2013; Brook and Rajagopalan 2010; Diez Roux et al. 2008; Künzli et al. 2005; Perez et al. 2015). Such studies observed higher pro-inflammatory cytokines following inhalation and deposition of PM in the lungs (Pope et al. 2016; Tan et al. 2000; Terashima et al. 1997a, 1997b; van Eeden et al. 2001) and the activation of coagulation and adhesion molecules (Baccarelli et al. 2007; Bind et al. 2012; O’Neill et al. 2007; Pope et al. 2016; Rückerl et al. 2006; Tsai et al. 2012), which could ultimately lead to increased leukocyte content within and vulnerability to rupture of atherosclerotic plaques (Brook and Rajagopalan 2010; Madjid et al. 2004; Ross 1999).
Although the inverse relationship between short-duration (i.e., 2- and 7-d mean) ambient exposures and leukocyte counts may be at odds with this suggestion, PM exposure may initiate pulmonary alveolar microvascular sequestration of monocytes and granulocytes (Goto et al. 2004; Terashima et al. 1999; Yatera et al. 2008), thereby reducing their concentrations in peripheral blood over the short term (Ghio et al. 2003; Yatera et al. 2008). Animal studies of monocytes and acute exposure also suggest that atherosclerotic plaques may recruit leukocytes from the circulation (Yatera et al. 2008). The inverse –leukocyte count associations with short-duration exposure in the present study are in contrast to null (Liao et al. 2005; Seaton et al. 1999) and positive (Schwartz 2001; Steinvil et al. 2008) epidemiologic associations observed in other contexts. However, they are consistent with observed inverse associations with short-duration exposure to in the Normative Aging Study (Zeka et al. 2006).
The characterization of PM–leukocyte associations in the compositional context is particularly relevant given the increasing availability of epigenomic biomarkers that are based on DNA extracted from peripheral blood with leukocyte proportions that can vary widely among participants. However, leukocyte cell types possess distinguishing patterns of DNAm, so measurements of methylation are driven in part by leukocyte composition (Jaffe and Irizarry 2014). Common practice is therefore to restrict measurement of DNAm to a single cell type (Chi et al. 2016b), to statistically adjust associations with DNAm for leukocyte proportions determined via cytometry as part of a complete blood count/differential, or in its absence, to adjust for DNAm-based estimates of T-cell, T-cell, NK cell, B-cell, monocyte, and granulocyte proportions (Houseman et al. 2012; Panni et al. 2016). Mindful of the PM–leukocyte compositional associations detected herein, causal diagrams (Greenland et al. 1999) may benefit from thoughtful consideration of their potential effects on causal association and mediation analyses (VanderWeele 2015; VanderWeele and Vansteelandt 2014) involving DNAm and other leukocyte-derived biomarkers. Indeed, leukocyte composition may itself be a mediator of PM–DNAm associations. As such, DNAm associations with —without control for leukocyte composition—may reflect mechanisms that involve inflammation, epigenetics, or both.
The present results are nevertheless limited by the variances of the observed association estimates. The analyses were weighted for attrition to avoid potential selection bias due to nonrandom loss to follow-up; however, the loss of bias came at the cost of precision (Cole and Hernán 2008). Furthermore, precision was influenced by technical, temporal, and biological variation of leukocyte count measurements. Participant blood samples were collected, processed, and analyzed by local laboratories across the United States using different automated hematology cell counters. Indeed, secular trends in methods of determining leukocyte count (Ruggiero et al. 2007) may have affected the precision or accuracy of association estimates. And while lack of adjustment for other cell (e.g., erythrocyte, platelet) counts capable of explaining some variation in leukocyte counts may have contributed to the precision of estimates observed herein, there also is evidence to suggest high within-laboratory reliability of leukocyte counts (Nieto et al. 1992) and robustness of study- and center-stratified, longitudinal model results to multiple methods of calendar date adjustment. Moreover, erythrocyte and platelet counts—plausible intermediates of PM–leukocyte count associations—were neither uniformly available nor necessarily appropriate candidates for statistical adjustment (Schisterman et al. 2009).
Additional limitations include error in estimated leukocyte proportions and PM concentrations. Although cytometrically determined leukocyte proportions for the cell types of interest were not available herein at participant visits with corresponding PM data, estimation of the T-cell, T-cell, NK cell, B-cell, monocyte, and granulocyte proportions at hand was associated with a low root mean square error (median rMSE: 8.2%, range: 5.4–11.6%) (Houseman et al. 2012; Koestler et al. 2013). Furthermore, the validity of spatially estimated daily estimates was demonstrated with an average prediction error and standardized prediction error near zero, a root mean square standardized near one, and a root mean square prediction error near the SE (Liao et al. 2006, 2007). Similarly, models for spatiotemporally estimated monthly mean and estimation performed well, with high squared Pearson correlations between excluded monthly observations and model predictions () in a 5- to 10-fold, out-of-sample cross-validation (Yanosky et al. 2014). Therefore, outcome and exposure measurement error were less likely to have biased observed associations.
Limitations aside, this longitudinal study observed that 1- and 12-month mean ambient concentrations were associated with higher leukocyte count. It is the first to do so in large, multi-ethnic and geographically diverse populations of women and men from two well-characterized cardiovascular disease cohorts. Furthermore, this study is the first to use compositional data analysis methods to estimate associations between ambient concentrations and leukocyte composition. Its analyses accounted for known relationships among proportions, thereby avoiding methodological biases inherent in conventional analyses that erroneously assume compositional data are independent. Results from them are therefore relatively well positioned to inform future causal analyses using leukocyte-derived biomarkers.
In conclusion, findings suggest that mid- to longer-duration ambient exposure to fine particulate matter () air pollution may induce subclinical, but epidemiologically important, inflammatory responses among racially, ethnically, and environmentally diverse U.S. populations in U.S. EPA Regions 1–10.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the Department of Health and Human Services (DHHS), National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) (contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I). Funding was also supported by NHLBI through the American Recovery and Reinvestment Act of 2009 (ARRA) 5RC2HL102419 and National Institute of Neurological Disorders and Stroke R01NS087541. Data from the ARIC study are available on request at https://www2.cscc.unc.edu/aric/distribution-agreements.
The WHI program is funded by the NHLBI through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. WHI-AS311 was supported by American Cancer Society award 125,299-RSG-13-100-01-CCE. All contributors to WHI science are listed at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf. Data from the WHI are available on request at https://www.whi.org/researchers/SitePages/Write%20a%20Paper.aspx.
This work was supported by the NIH/National Institute of Environmental Health Sciences (NIEHS) grant R01-ES020836 (L.H., A.B., E.A.W.), NHLBI contract HHSN268201100046C (K.N.C.), NIEHS grant R01-ES017794 (E.A.W.), NHLBI National Research Service Award T32-HL007055 (R.G.), NIEHS National Research Service Award T32-ES007018 (K.M.H.), and National Cancer Institute grant R25-CA094880 (K.M.J.).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5360).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Adar SD, Sheppard L, Vedal S, Polak JF, Sampson PD, Diez Roux AV, et al. . 2013. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the Multi-Ethnic Study of Atherosclerosis and Air Pollution. PLoS Med 10(4):e1001430, PMID: 23637576, 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison J. 1982. The statistical analysis of compositional data. J R Stat Soc Series B Stat Methodol 44(2):139–177, 10.1111/j.2517-6161.1982.tb01195.x. [DOI] [Google Scholar]
- Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. . 2003. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 13(suppl 9):S5–S17, PMID: 14575938, 10.1016/S1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- ARIC Investigators. 1989. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol 129:687–702, PMID: 2646917, 10.1093/oxfordjournals.aje.a115184. [DOI] [PubMed] [Google Scholar]
- Assimes T, Tsao P, Absher D, Horvath S. 2018. BA23 – integrative genomics and risk of CHD and related phenotypes in the Women’s Health Initiative. https://www.whi.org/researchers/data/WHIStudies/StudySites/BA23/pages/home.aspx [accessed 20 April 2018].
- Azur MJ, Stuart EA, Frangakis C, Leaf PJ. 2011. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 20(1):40–49, PMID: 21499542, 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Zanobetti A, Martinelli I, Grillo P, Hou L, Giacomini S, et al. . 2007. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost 5(2):252–260, PMID: 17083648, 10.1111/j.1538-7836.2007.02300.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu M, Benoit L, Abaga S, Kappeler PM, Charpentier EMJ. 2017. Mind the cell: seasonal variation in telomere length mirrors changes in leucocyte profile. Mol Ecol 26(20):5603–5613, PMID: 28817217, 10.1111/mec.14329. [DOI] [PubMed] [Google Scholar]
- Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. 2013. Time series regression studies in environmental epidemiology. Int J Epidemiol 42(4):1187–1195, PMID: 23760528, 10.1093/ije/dyt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti P. 2018. As311 - DNA methylation measured in prospectively collected blood samples and risk of bladder cancer among post-menopausal women. https://www.whi.org/researchers/data/WHIStudies/StudySites/AS311/Pages/home.aspx [accessed 20 April 2018].
- Bind M-A, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, et al. . 2012. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology 23(2):332–340, PMID: 22237295, 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. 2010. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep 12(5):291–300, PMID: 20617466, 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. . 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. . 2009. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54(3):659–667, PMID: 19620518, 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Giles WH, Croft JB. 2001. White blood cell count: an independent predictor of coronary heart disease mortality among a national cohort. J Clin Epidemiol 54(3):316–322, PMID: 11223329, 10.1016/s0895-4356(00)00296-1. [DOI] [PubMed] [Google Scholar]
- Chastin SF, Palarea-Albaladejo J, Dontje ML, Skelton DA. 2015. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS One 10(10):e0139984, PMID: 26461112, 10.1371/journal.pone.0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-C, Schwartz J. 2008. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ Health Perspect 116(5):612–617, PMID: 18470293, 10.1289/ehp.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi GC, Hajat A, Bird CE, Cullen MR, Griffin BA, Miller KA, et al. . 2016a. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ Health Perspect 124(12):1840–1847, PMID: 27138533, 10.1289/EHP199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi GC, Liu Y, MacDonald JW, Barr RG, Donohue KM, Hensley MD, et al. . 2016b. Long-term outdoor air pollution and DNA methylation in circulating monocytes: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health 15(1):119, PMID: 27903268, 10.1186/s12940-016-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang K-J, Yan Y-H, Chiu S-Y, Cheng T-J. 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 68(1):64–68, PMID: 20833756, 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- Cochran WG. 1954. The combination of estimates from different experiments. Biometrics 10(1):101–129, 10.2307/3001666. [DOI] [Google Scholar]
- Cole SR, Hernán MA. 2008. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168(6):656–664, PMID: 18682488, 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, Peto R. 1998. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279(18):1477–1482, PMID: 9600484, 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188, PMID: 3802833, 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. . 2017. Air pollution and mortality in the Medicare population. N Engl J Med 376(26):2513–2522, PMID: 28657878, 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, et al. . 2008. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 167(6):667–675, PMID: 18227099, 10.1093/aje/kwm359. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. . 2001. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345(2):99–106, PMID: 11450679, 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Zeger SL, Samet JM. 2002. On the use of generalized additive models in time-series studies of air pollution and health. Am J Epidemiol 156(3):193–203, PMID: 12142253, 10.1093/aje/kwf062. [DOI] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. 2006. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect 114(7):992–998, PMID: 16835049, 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, Barceló-Vidal C. 2003. Isometric logratio transformations for compositional data analysis. Math Geol 35(3):279–300, 10.1023/A:1023818214614. [DOI] [Google Scholar]
- Elo IT. 2009. Social class differentials in health and mortality: patterns and explanations in comparative perspective. Annu Rev Sociol 35(1):553–572, 10.1146/annurev-soc-070308-115929. [DOI] [Google Scholar]
- Emmerechts J, Jacobs L, Van Kerckhoven S, Loyen S, Mathieu C, Fierens F, et al. . 2012. Air pollution-associated procoagulant changes: the role of circulating microvesicles. J Thromb Haemost 10(1):96–106, PMID: 22066779, 10.1111/j.1538-7836.2011.04557.x. [DOI] [PubMed] [Google Scholar]
- Fairclough SJ, Dumuid D, Taylor S, Curry W, McGrane B, Stratton G, et al. . 2017. Fitness, fatness and the reallocation of time between children’s daily movement behaviours: an analysis of compositional data. Int J Behav Nutr Phys Act 14(1):64, PMID: 28486972, 10.1186/s12966-017-0521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, Boere AJF, Leseman DLAC, Dormans JAMA, Sandström T, Salonen RO, et al. . 2005. Effects of particulate matter on the pulmonary and vascular system: time course in spontaneously hypertensive rats. Part Fibre Toxicol 2(1):2, PMID: 15813961, 10.1186/1743-8977-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. 2003. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhal Toxicol 15(14):1465–1478, PMID: 14648359, 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- Gong H Jr, Linn WS, Terrell SL, Anderson KR, Clark KW, Sioutas C, et al. . 2004. Exposures of elderly volunteers with and without chronic obstructive pulmonary disease (COPD) to concentrated ambient fine particulate pollution. Inhal Toxicol 16(11–12):731–744, PMID: 16036744, 10.1080/08958370490499906. [DOI] [PubMed] [Google Scholar]
- Gordon T, Nadziejko C, Schlesinger R, Chen LC. 1998. Pulmonary and cardiovascular effects of acute exposure to concentrated ambient particulate matter in rats. Toxicol Lett 96–97:285–288, PMID: 9820679, 10.1016/S0378-4274(98)00084-8. [DOI] [PubMed] [Google Scholar]
- Goto Y, Ishii H, Hogg JC, Shih C-H, Yatera K, Vincent R, et al. . 2004. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med 170(8):891–897, PMID: 15256391, 10.1164/rccm.200402-235OC. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48, PMID: 9888278, 10.1097/00001648-199901000-00008. [DOI] [PubMed] [Google Scholar]
- Hajat A, Hsia C, O’Neill MS. 2015. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep 2(4):440–450, PMID: 26381684, 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LK, Grimm RH Jr, Neaton JD. 1990. The relationship of white blood cell count to other cardiovascular risk factors. Int J Epidemiol 19(4):881–888, PMID: 2084016, 10.1093/ije/19.4.881. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Robins JM. 2004. A structural approach to selection bias. Epidemiology 15(5):615–625, PMID: 15308962, 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. . 2012. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC bioinformatics 13(1):86, PMID: 22568884, 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ Jr.. 2016. Selection bias due to loss to follow up in cohort studies. Epidemiology 27(1):91–97, PMID: 26484424, 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J-F, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, et al. . 1999. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science 286(5441):952–954, PMID: 10542149, 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- Huang W-H, Yen T-H, Chan M-J, Su Y-J. 2014. Impact of environmental particulate matter and peritoneal dialysis-related infection in patients undergoing peritoneal dialysis. Medicine (Baltimore) 93(25):e149, PMID: 25437027, 10.1097/MD.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L, Emmerechts J, Mathieu C, Hoylaerts MF, Fierens F, Hoet PH, et al. . 2010. Air pollution-related prothrombotic changes in persons with diabetes. Environ Health Perspect 118(2):191–196, PMID: 20123602, 10.1289/ehp.0900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AE, Irizarry RA. 2014. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome biology 15(2):R31, PMID: 24495553, 10.1186/gb-2014-15-2-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat GC, Kim MY, Manson JE, Lessin L, Lin J, Wassertheil-Smoller S, et al. . 2017. White blood cell count and total and cause-specific mortality in the Women’s Health Initiative. Am J Epidemiol 186(1):63–72, PMID: 28369251, 10.1093/aje/kww226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Ledbetter AD, Hauser R, Christiani DC, McGee J, et al. . 2002. Temporal association between pulmonary and systemic effects of particulate matter in healthy and cardiovascular compromised rats. J Toxicol Environ Health Part A 65(20):1545–1569, PMID: 12396868, 10.1080/00984100290071667. [DOI] [PubMed] [Google Scholar]
- Koestler DC, Christensen B, Karagas MR, Marsit CJ, Langevin SM, Kelsey KT, et al. . 2013. Blood-based profiles of DNA methylation predict the underlying distribution of cell types: a validation analysis. Epigenetics 8(8):816–826, PMID: 23903776, 10.4161/epi.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, et al. . 2005. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect 113(2):201–206, PMID: 15687058, 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. 2001. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and white men and women: Atherosclerosis Risk in Communities study. Am J Epidemiol 154(8):758–764, PMID: 11590089, 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- Liao D, Heiss G, Chinchilli VM, Duan Y, Folsom AR, Lin H-M, et al. . 2005. Association of criteria pollutants with plasma hemostatic//inflammatory markers: a population-based study. J Expo Anal Environ Epidemiol 15(4):319–328, PMID: 15536489, 10.1038/sj.jea.7500408. [DOI] [PubMed] [Google Scholar]
- Liao D, Peuquet DJ, Duan Y, Whitsel EA, Dou J, Smith RL, et al. . 2006. GIS approaches for the estimation of residential-level ambient PM concentrations. Environ Health Perspect 114(9):1374–1380, PMID: 16966091, 10.1289/ehp.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Peuquet DJ, Lin H-M, Duan Y, Whitsel EA, Smith RL, et al. . 2007. National kriging exposure estimation: Liao et al. respond. Environ Health Perspect 115(7):A338–A339, 10.1289/ehp.10205R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjid M, Awan I, Willerson JT, Casscells SW. 2004. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol 44(10):1945–1956, PMID: 15542275, 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. . 2002. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med 347(10):716–725, PMID: 12213942, 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. . 2005. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med 165(5):500–508, PMID: 15767524, 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- McCullough SD, Dhingra R, Fortin MC, Diaz-Sanchez D. 2017. Air pollution and the epigenome: a model relationship for the exploration of toxicoepigenetics. Curr Opin Toxicol 6:18–25, 10.1016/j.cotox.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. . 2007. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356(5):447–458, PMID: 17267905, 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Mills NL, Törnqvist H, Gonzalez MC, Vink E, Robinson SD, Söderberg S, et al. . 2007. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med 357(11):1075–1082, PMID: 17855668, 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, et al. . 2005. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112(25):3930–3936, PMID: 16365212, 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Mosley TH Jr, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, et al. . 2005. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology 64(12):2056–2062, PMID: 15985571, 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- NCDC (National Centers for Environmental Information). 2019. Global surface summary of the day–GSOD. https://data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.ncdc:C00516 [accessed 12 December 2019].
- Neupane R, Jin X, Sasaki T, Li X, Murohara T, Cheng XW. 2019. Immune disorder in atherosclerotic cardiovascular disease—clinical implications of using circulating T-cell subsets as biomarkers. Circ J 83(7):1431–1438, PMID: 31092769, 10.1253/circj.CJ-19-0114. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Szklo M, Folsom AR, Rock R, Mercuri M. 1992. Leukocyte count correlates in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 136(5):525–537, PMID: 1442716, 10.1093/oxfordjournals.aje.a116530. [DOI] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, et al. . 2007. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med 64(6):373–379, PMID: 17182639, 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panni T, Mehta AJ, Schwartz JD, Baccarelli AA, Just AC, Wolf K, et al. . 2016. A genome-wide analysis of DNA methylation and fine particulate matter air pollution in three study populations: KORA F3, KORA F4, and the Normative Aging Study. Environ Health Perspect 124(7):983–990, PMID: 26731791, 10.1289/ehp.1509966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp AC, Hatzakis H, Bracey A, Wu KK. 1989. ARIC hemostasis study–I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost 61(1):15–19, PMID: 2526384, 10.1055/s-0038-1646519. [DOI] [PubMed] [Google Scholar]
- Parker JD, Kravets N, Vaidyanathan A. 2018. Particulate matter air pollution exposure and heart disease mortality risks by race and ethnicity in the United States. 1997 to 2009 National Health Interview Survey with mortality follow-up through 2011. Circulation 137(16):1688–1697, PMID: 29237717, 10.1161/CIRCULATIONAHA.117.029376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowsky-Glahn V, Egozcue JJ, Tolosana-Delgado R. 2015. Linear models. In: Modelling and Analysis of Compositional Data. Chichester, UK: John Wiley & Sons, 149–171. [Google Scholar]
- Peng RD, Dominici F, Louis TA. 2006. Model choice in time series studies of air pollution and mortality. J R Stat Soc Ser A Stat Soc 169(2):179–203, 10.1111/j.1467-985X.2006.00410.x. [DOI] [Google Scholar]
- Perez L, Wolf K, Hennig F, Penell J, Basagaña X, Foraster M, et al. . 2015. Air pollution and atherosclerosis: a cross-sectional analysis of four European cohort studies in the ESCAPE study. Environ Health Perspect 123(6):597–605, PMID: 25625785, 10.1289/ehp.1307711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti DB, Kipp H. 1986. The leukocyte count: associations with intensity of smoking and persistence of effect after quitting. Am J Epidemiol 123(1):89–95, PMID: 3940445, 10.1093/oxfordjournals.aje.a114227. [DOI] [PubMed] [Google Scholar]
- Pope CA III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. 2016. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119(11):1204–1214, PMID: 27780829, 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA III, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, et al. . 2004. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 112(3):339–345, PMID: 14998750, 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M. 2007. Cardiovascular effects of fine particulate matter components in highway patrol officers. Inhal Toxicol 19(suppl 1):99–105, PMID: 17886057, 10.1080/08958370701495238. [DOI] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, et al. . 2004. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med 169(8):934–940, PMID: 14962820, 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Ross R. 1999. Atherosclerosis—an inflammatory disease. N Engl J Med 340(2):115–126, PMID: 9887164, 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, et al. . 2006. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med 173(4):432–441, PMID: 16293802, 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Metter EJ, Cherubini A, Maggio M, Sen R, Najjar SS, et al. . 2007. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 49(18):1841–1850, PMID: 17481443, 10.1016/j.jacc.2007.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandström T, Holgate ST, et al. . 1999. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 159(3):702–709, PMID: 10051240, 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. 2009. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20(4):488–495, PMID: 19525685, 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. 2001. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect 109(suppl 3):405–409, PMID: 11427390, 10.2307/3434788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Weiss ST. 1991. Host and environmental factors influencing the peripheral blood leukocyte count. Am J Epidemiol 134(12):1402–1409, PMID: 1776614, 10.1093/oxfordjournals.aje.a116045. [DOI] [PubMed] [Google Scholar]
- Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, et al. . 1999. Particulate air pollution and the blood. Thorax 54(11):1027–1032, PMID: 10525563, 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhof M, Janssen NAH, Strak M, Hoek G, Gosens I, Mudway IS, et al. . 2014. Air pollution exposure affects circulating white blood cell counts in healthy subjects: the role of particle composition, oxidative potential and gaseous pollutants—the RAPTES project. Inhal Toxicol 26(3):141–165, PMID: 24517839, 10.3109/08958378.2013.861884. [DOI] [PubMed] [Google Scholar]
- Steinvil A, Kordova-Biezuner L, Shapira I, Berliner S, Rogowski O. 2008. Short-term exposure to air pollution and inflammation-sensitive biomarkers. Environ Res 106(1):51–61, PMID: 17915210, 10.1016/j.envres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Stolwijk AM, Straatman H, Zielhuis GA. 1999. Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Community Health 53(4):235–238, PMID: 10396550, 10.1136/jech.53.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart EA, Azur M, Frangakis C, Leaf P. 2009. Multiple imputation with large data sets: a case study of the Children’s Mental Health Initiative. Am J Epidemiol 169(9):1133–1139, PMID: 19318618, 10.1093/aje/kwp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, et al. . 2000. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med 161(4 Pt 1):1213–1217, PMID: 10764314, 10.1164/ajrccm.161.4.9904084. [DOI] [PubMed] [Google Scholar]
- Terashima T, Klut ME, English D, Hards J, Hogg JC, van Eeden SF. 1999. Cigarette smoking causes sequestration of polymorphonuclear leukocytes released from the bone marrow in lung microvessels. Am J Respir Cell Mol Biol 20(1):171–177, PMID: 9870931, 10.1165/ajrcmb.20.1.3276. [DOI] [PubMed] [Google Scholar]
- Terashima T, Wiggs B, English D, Hogg JC, van Eeden SF. 1997a. Phagocytosis of small carbon particles (PM10) by alveolar macrophages stimulates the release of polymorphonuclear leukocytes from bone marrow. Am J Respir Crit Care Med 155(4):1441–1447, PMID: 9105091, 10.1164/ajrccm.155.4.9105091. [DOI] [PubMed] [Google Scholar]
- Terashima T, Wiggs B, English D, Hogg JC, van Eeden SF. 1997b. The effect of cigarette smoking on the bone marrow. Am J Respir Crit Care Med 155(3):1021–1026, PMID: 9116981, 10.1164/ajrccm.155.3.9116981. [DOI] [PubMed] [Google Scholar]
- Törnqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, et al. . 2007. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med 176(4):395–400, PMID: 17446340, 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Tsai D-H, Amyai N, Marques-Vidal P, Wang J-L, Riediker M, Mooser V, et al. . 2012. Effects of particulate matter on inflammatory markers in the general adult population. Part Fibre Toxicol 9:24, PMID: 22769230, 10.1186/1743-8977-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2017. What are the air quality standards for PM? https://www3.epa.gov/region1/airquality/pm-aq-standards.html [accessed 20 April 2018].
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. . 2001. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am J Respir Crit Care Med 164(5):826–830, PMID: 11549540, 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ. 2015. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press. [Google Scholar]
- VanderWeele TJ, Vansteelandt S. 2014. Mediation analysis with multiple mediators. Epidemiol Methods 2(1):95–115, PMID: 25580377, 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Möhlenkamp S, et al. . 2015. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med 72(9):656–663, PMID: 26163546, 10.1136/oemed-2014-102800. [DOI] [PubMed] [Google Scholar]
- Whitsel EA. 2018. Epigenetic Mechanisms of PM-Mediated CVD Risk. Project no. 4R01ES020836-05. https://projectreporter.nih.gov/project_info_description.cfm?aid=9054857&icde=40216248&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball= [accessed 20 April 2018].
- Whitsel EA, Quibrera PM, Smith RL, Catellier DJ, Liao D, Henley AC, et al. . 2006. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov 3:8, PMID: 16857050, 10.1186/1742-5573-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G. 2004. Accuracy and repeatability of commercial geocoding. Am J Epidemiol 160(10):1023–1029, PMID: 15522859, 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- Women’s Health Initiative Study Group. 1998. Design of the Women’s Health Initiative Clinical Trial and Observational Study. The Women’s Health Initiative Study Group. Control Clin Trials 19(1):61–109, PMID: 9492970, 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. . 2014. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health 13:63, PMID: 25097007, 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatera K, Hsieh J, Hogg JC, Tranfield E, Suzuki H, Shih C-H, et al. . 2008. Particulate matter air pollution exposure promotes recruitment of monocytes into atherosclerotic plaques. Am J Physiol Heart Circ Physiol 294(2):H944–H953, PMID: 18083905, 10.1152/ajpheart.00406.2007. [DOI] [PubMed] [Google Scholar]
- Zeka A, Sullivan JR, Vokonas PS, Sparrow D, Schwartz J. 2006. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int J Epidemiol 35(5):1347–1354, PMID: 16844771, 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
- Zhao J, Gao Z, Tian Z, Xie Y, Xin F, Jiang R, et al. . 2013. The biological effects of individual-level PM2.5 exposure on systemic immunity and inflammatory response in traffic policemen. Occup Environ Med 70(6):426–431, PMID: 23322918, 10.1136/oemed-2012-100864. [DOI] [PubMed] [Google Scholar]
- Zhong J, Agha G, Baccarelli AA. 2016. The role of DNA methylation in cardiovascular risk and disease. methodological aspects, study design, and data analysis for epidemiological studies. Circ Res 118(1):119–131, PMID: 26837743, 10.1161/CIRCRESAHA.115.305206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.