Abstract
Background & Aims:
In some patients, the type 3 achalasia (A3) motor pattern may be an effect of chronic use of high-dose opioids. No motor findings have been identified to differentiate opioid-induced A3 (OA3) from idiopathic A3 (IA3). We investigated whether OA3 could be distinguished from IA3 on the basis of differences in esophageal motor responses to amyl nitrite, cholecystokinin, or atropine.
Methods:
We performed a retrospective study of patients who received pharmacologic provocation during esophageal high-resolution manometry from 2007 through 2017 at a tertiary referral center. We identified 26 patients with IA3 (9 women; mean age, 68±13 years) and 24 patients with OA3 (15 women; mean age, 59±10 years). We compared pressure topography metrics during deglutition and after administration of amyl nitrite, cholecystokinin, or atropine between patients with OA3 vs IA3.
Results:
Amyl nitrite induced a similar relaxation response in both groups, but the rebound contraction of the lower esophageal sphincter during amyl nitrite recovery, and the paradoxical esophageal contraction during the first phase of cholecystokinin response, were both significantly attenuated in patients with OA3. The second phase of cholecystokinin response in patients with OA3 was 100% relaxation, when present, in contrast to only 26% of patients with IA3. There was no significant difference between groups in inhibition of lower esophageal sphincter tone or esophageal body contractility by cholinergic receptor blockade.
Conclusions:
Nearly half of patients with an A3 pattern of dysmotility are chronic, daily users of opioids with manometry patterns indistinguishable from those of patients with IA3. Patients with OA3 differ from patients with IA3 in responses to amyl nitrite and cholecystokinin. These findings might be used to identify patients with dysmotility resulting from opiate use.
Keywords: LES, HRM, narcotics, dysphagia, peristalsis
BACKGROUND
Previous studies have suggested that chronic opioid therapy can induce patterns of esophageal dysmotility1, including severe disturbances typically associated with idiopathic achalasia2. The Chicago Classification type-3 achalasia (A3) is significantly associated with high-dose chronic opioid exposure3. Patients with idiopathic achalasia type-3 (IA3) are typically considered to require invasive treatment modalities such as long esophageal myotomy (laparoscopic or POEM) for adequate palliation of symptoms. Such therapies result in an irreversibly flaccid distal esophagus and permanent loss of the lower esophageal sphincter (LES) anti-reflux barrier. Idiopathic achalasia is currently an irreversible condition, whereas opioid-induced achalasia type-3 (OA3) may resolve with modification of narcotic therapy4.
The function of excitatory and inhibitory neural pathways in esophageal motor disorders can be investigated by the use of pharmacologic agents such as amyl nitrite (AN), cholecystokinin-octapeptide (CCK) and atropine (ATR)5–8. These agents have rapid onset and relatively short duration of action and can be safely administered during manometric investigation. AN inhalation produces a rapid relaxation of smooth muscle. When the AN-induced integrated relaxation pressure (IRP) falls significantly below the deglutitive IRP, this demonstrates that endogenous LES smooth muscle relaxation is impaired8. Pharmacologic administration of CCK has competing excitatory and inhibitory effects in the esophagus. The inhibitory neural effects are usually predominant, so the excitatory muscular effects are unveiled when such inhibitory innervation is impaired6. The pharmacologic response to ATR has demonstrated the persistence of excitatory cholinergic-mediated tone and contractility in some patients with esophageal dysmotility such as achalasia5. Therefore, at the Medical College of Wisconsin (MCW), we have historically used AN, CCK and ATR to investigate the integrity of excitatory and inhibitory neural pathways in patients with manometric evidence of major motor disorders, such as achalasia.
The potential inhibitory or excitatory pathway impairments in the OA3 may be different from those in IA3. Therefore, we hypothesized that pharmacologic testing using AN, CCK and ATR may show distinctive esophageal motor responses that differentiate OA3 from IA3 and provide relevant mechanistic information about their pathophysiology. Such differences might prove useful in identifying occult opiate use in patients with symptomatic dysmotility or determining that motor disturbances would be unlikely to resolve with cessation of opiate use.
METHODS:
This study is a retrospective analysis of clinical manometry studies aimed to compare deglutitive pressure metrics and esophageal motor responses to AN, CCK and ATR between OA3 and IA3. The investigational review board of the MCW approved the study protocol.
Study Population:
In a cohort of 2705 patients who underwent esophageal HRM from 2007–2017 in a tertiary referral center, 51 patients with A3 motor pattern were identified by the consensus agreement. One OA3 patient was excluded from analysis due to absence of pharmacologic challenge during HRM. Then medical records of the cohort were carefully reviewed to identify opioid users. Patients with a history of long-acting opioid agents with equal or greater potency than morphine for at least 90 days, at the time of HRM, were considered chronic daily opioid users as previously described3. Four patients had only intermittent short-acting opioid use and were included in the idiopathic cohort. The duration of opioid intake was recorded based on the first documentation of opioid prescription in the electronic health record system until the date of HRM. The morphine milligram equivalent daily dose (MMED) of opioids, based on standard conversion factors of equianalgesic charts were recorded3.
Medical records were reviewed to determine the outcome of therapy for A3 patients. Laparoscopic myotomy and pneumatic dilation (≥ 30 mm) were considered permanent LES-ablative treatments. Non-ablative therapies included weaning of narcotics (when applicable), smooth muscle relaxants (nitrates, calcium channel blockers, anticholinergics), botulinum toxin injection, dilation ≤ 20 mm, and/or dietary modifications.
Esophageal High-Resolution Manometry (HRM):
The HRM catheter contained 36 circumferential solid-state pressure sensors, spaced 1 cm apart (Medtronic, Minneapolis, MN). The HRM data were reviewed to extract topographic pressure metrics of integrated relaxation pressure (IRP), distal latency (DL), distal contractile integral (DCI), maximum intrabolus pressure (IBP) and conventional parameters such as deglutitive EGJ relaxation nadir, basal expiratory EGJ and gastric pressures. The four second IRP threshold of 15 mmHg was considered the cut off threshold for the normal range in the Manoview® system9, 10. IRP, EGJ basal and deglutitive nadir pressures were referenced to gastric pressure.
Pharmacologic interrogation was always performed at the end of the standard protocol while the patient remained in the recumbent position. Heart rate and blood pressure were monitored. For AN administration, the registered nurse held crushed ampules of 0.3ml AN (James Alexander Corporation, Blairstown, NJ) below the nares of patients, and instructed patients to sniff the vapor four times. An intravenous line was established, and five micrograms of Cholecystokinin-octapeptide (Kinevac ®, Bracco Diagnostics, NJ) was mixed with five milliliters of sterile water to a concentration of 1 mcg/ml. CCK always was injected at the dose of 40 ng/kg more than 5 minutes after AN inhalation, and 10 ml of normal saline was used to flush the IV line. No wet swallows were given, but patients could swallow ad lib after AN and CCK administration. Once the LES pressure returned to the stable baseline following CCK, atropine sulfate (American Regent, Shirley, NY) was given intravenously at the dose of 12 mcg/kg. Once the heart rate increased after atropine administration, three wet swallows in the supine position were obtained. None of the patients in our laboratory required further medical care due to side effects from these pharmacologic agents during the study period.
Amyl Nitrite (AN) Response:
The first 60s after AN inhalation is usually the relaxation phase of response and the subsequent 120s contains the recovery period from AN. The onset and duration of relaxation were measured from 50% drop to 50% recovery of the LES basal tone. The highest amplitude of LES tone during the recovery phase (excluding 20s of post-deglutitive after-contraction if present) were recorded. We defined the “relaxation gain” as the deglutitive IRP minus the AN-induced relaxation IRP, and the “rebound contraction” as the LES peak expiratory pressure during recovery minus AN-induced relaxation LES nadir pressure as previously described8.
Cholecystokinin (CCK) Response:
The CCK responses were categorized as formerly described11: 1) Phase-I started ~ 10–30s after the CCK injection showing phasic esophageal body contractions associated with either relaxation or contractile LES motor response. The DCI was measured over a 30s window during phase-1 response. The highest amplitude of end-expiratory LES tone during the phase-1 minus basal LES nadir expiratory pressure before CCK administration was recorded as the “paradoxical contraction” (excluding 20s of post-deglutitive after-contraction if present). 2) Phase-II of the response was 30–100s after the CCK injection; this was associated with esophageal shortening of more than 2cm and crural diaphragm inhibition. CCK response typically terminated by a strong after-contraction similar to that of a transient LES relaxation event11. The IRP during esophageal shortening of ≥3 cm and DCI of the terminating event of phase-II CCK response were measured.
Atropine (ATR) Response:
The participant’s heart rate was monitored after intravenous atropine was given to observe for relative anticholinergic related tachycardia. Baseline LES pressure and median deglutitive pressure metrics under anticholinergic effects were measured.
Statistical Analysis:
We compared the deglutitive and pharmacologic agent-induced pressure metrics between the two groups of OA3 and IA3. Continuous variables are shown as median and interquartile range (IQR) and compared using Wilcoxon Rank-sum tests. Categorical variables are shown as n (%) and compared using the Chi square and Fisher’s exact test. The range of pharmacologic responses of both groups were reviewed to identify threshold values with greater than or equal to 95% specificity. The corresponding positive likelihood ratio (PLR) to the identified threshold was computed (PLR= Sensitivity/1-specificity).
RESULTS
We identified 24 OA3 (15F, 59 ± 10 years) and 26 IA3 patients (9F, 68 ± 13 years). The OA3 patients were significantly younger and predominantly female (Table 1). They were less likely to have liquid dysphagia or evidence of esophageal lumen dilation on esophagram studies. The endoscopic and biopsy findings in 49 patients were available prior to HRM. None of the patients had an esophageal stricture or esophageal mucosal eosinophilia. The deglutitive pressure metrics were not significantly different between the two groups (Table 2).
Table 1. Clinical characteristics of patients with type-3 achalasia divided by chronic daily opioid exposure into idiopathic (IA3) and opioid-induced (OA3) groups.
Continuous variables are shown as median, interquartile range and compared using Wilcoxon Rank-Sum tests. Categorical variables are shown as n (%) and compared using Fisher exact test.
| Clinical Characteristics | |||
|---|---|---|---|
| Demographics | IA3 (N=26) |
OA3 (N=24) |
P value |
| Female | 9 (35%) | 1 5 (63%) | 0.05 |
| Caucasian | 22 (85%) | 22 (92%) | 0.8 |
| Age | 72 (60, 76) | 57 (53, 66) | 0.006* |
| Body Mass Index | 28 (25, 31) | 28 (25, 35) | 0.9 |
| Medical History | |||
| Opioid Exposure Duration (m) | 0 (0, 0) | 50 (32, 89) | <0.001* |
| Morphine Equivalent Daily | 0 (0, 0) | 169 (90, 355) | <0.001* |
| Cancer | 7 (27%) | 2 (8%) | 0.09 |
| Cardiac Disease | 9 (35%) | 7 (29%) | 0.8 |
| Pulmonary Disease | 4 (1 5%) | 3 (13%) | 0.8 |
| Connective Tissue Disease | 4 (1 5%) | 6 (25%) | 0.5 |
| Fibromyalgia | 0 (0%) | 5 (21%) | 0.01* |
| Symptom Presentation | |||
| Symptom Duration (months) | 36 (11, 60) | 31 (12,60) | 0.8 |
| Weight Loss (lbs) | 0(0, 10) | 0 (0, 3) | 0.3 |
|
2 (8%) 22 (88%) |
12 (50%) 10 (42%) |
|
|
8 (32%) 7 (28%) |
6 (25%) 7 (29%) |
|
|
6 (24%) 9 (36%) |
9 (38%) 6 (25%) |
|
| Esophagram | N=20 | N=18 | |
| Hiatal Hernia | 7 (35%) | 1 (6%) | 0.03* |
| EGJ Narrowing | 13 (65%) | 6 (33%) | 0.05 |
|
4 (20%) 5 (25%) 0 (0%) |
0 (0%) 0 (0%) 0 (0%) |
|
|
0 (0%) 3 (1 5%) 16 (80%) |
1 (6%) 3 (17%) 12 (67%) |
|
| EGD | N=24 | N=24 | |
| Barrett’s esophagus | 1 (4%) | 1 (4%) | 1.0 |
| Esophageal Diverticulum | 1 (4%) | 1 (4%) | 1.0 |
| Esophageal Stricture | 0 (0%) | 0 (0%) | 1.0 |
Table 2. Esophageal pressure topography characteristics in patients with type-3 achalasia divided by chronic daily opioid exposure into idiopathic (IA3) and opioid-induced (OA3) subgroups.
The data are shown as median and interquartile range and all pressures are in mmHg.
| Esophageal Pressure Topography Characteristics | |||
|---|---|---|---|
| Pressure Metrics | IA3 (N=26) |
OA3 (N=24) |
P value |
| Integrated Relaxation Pressure | 28 (23, 38) | 29 (22, 38) | 0.7 |
| Deglutitive Nadir Pressure | 26 (17, 33) | 22 (18, 27) | 0.2 |
| Intrabolus Pressure | 25 (20, 33) | 25 (20, 30) | 0.9 |
| Distal Contractile Integral (mmHg.s.cm) | 1894 (1283,5795) | 3075 (1098,5575) | 0.7 |
| Distal Latency (s) | 4.3 (3.7, 5) | 4 (3.5,4.4) | 0.1 |
| Contraction Front Velocity (cm/s) | 9 (8, 9) | 9 (8,9) | 0.5 |
| Basal LES Expiratory Pressure | 35 (26, 51) | 31 (26, 45) | 0.3 |
| Basal Gastric Pressure | 9 (7, 11) | 10 (7, 14) | 0.5 |
| Basal Esophageal Pressure | 5 (2, 8) | 4 (0, 9) | 0.4 |
| Frequency Pressure Pattern | |||
| Failed Peristalsis | 0 (0, 30) | 0 (0, 20) | 1.0 |
| Pan-Pressurization | 0 (0, 30) | 0 (0, 10) | 1.0 |
| Premature Contraction | 50 (20, 90) | 80 (60, 90) | 0.3 |
| Rapid Contraction | 40 (20, 80) | 50 (20, 70) | 0.5 |
Amyl Nitrite
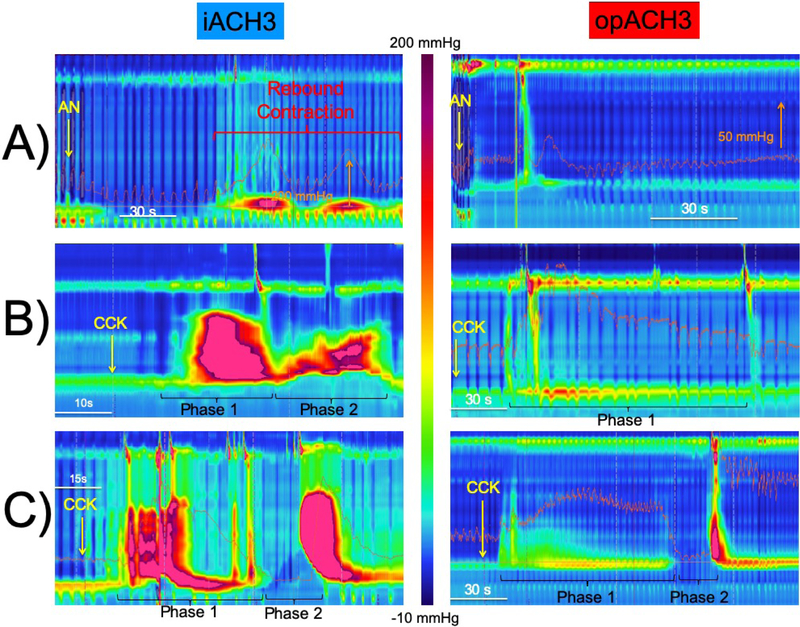
The duration and amplitude of thoracic pressure drop during AN inhalation, and the relaxation and recovery lag times after AN were similar across IA3 and OA3 groups, indicating similar systemic AN delivery in both groups (Table 3). AN induced a similar profound LES relaxation response in both groups to a residual AN-induced median IRP of 9 and 6 mmHg in the OA3 and IA3 patients respectively (p=0.3). This resulted in similar relaxation gains in both groups (Table 3). However, an exaggerated rebound LES contraction (≥50 mmHg) was significantly more common in IA3 (79%) than OA3 (26%) patients (p <0.001, Figure 1A).
Table 3. Esophageal pressure topography characteristics of pharmacologic interrogation in patients with type-3 achalasia divided by chronic daily opioid exposure into idiopathic (IA3) and opioid-induced (OA3) subgroups.
The data are shown as median and interquartile range. Integrated relaxation pressure (IRP), distal contractile integral (DCI).
| Esophageal Pressure Topography | |||
|---|---|---|---|
| Amyl Nitrite (AN) | IA3 (N=24) |
OA3 (N=23) |
P value |
| Inhalation Duration (s) | 10 (9, 15) | 11 (8, 14) | 0.4 |
| Inhalation Pressure Drop (s) | 8 (7, 11) | 10 (8, 12) | 0.3 |
| Onset Relaxation (s) | 15 (6, 19) | 16 (10, 20) | 0.7 |
| Relaxation Duration (s) | 52 (41, 55) | 42 (28, 50) | 0.04* |
| Relaxation IRP (mmHg) | 6 (3, 10) | 9 (5, 13) | 0.3 |
| Relaxation Gain (mmHg) | 20 (16, 34) | 18 (14, 29) | 1.0 |
| Recovery Swallow IRP (mmHg) | 49 (40, 82) | 44 (34, 53) | 0.1 |
| Rebound Contraction | 83 (51, 167) | 43 (29, 60) | <0.001* |
| Cholecystokinin (CCK) Phase 1 | N=23 | N=19 | |
| Nadir LES Pressure (mmHg) | 55 (32, 79) | 36 (16, 76) | 0.1 |
| Esophageal DCI (mmHg.cm.s) | 12055 (3135, 26596) | 1095 (436, 6205) | <0.001* |
| Paradoxical LES Contraction (mmHg) | 231 (111, 323) | 88 (35, 100) | <0.001* |
| Cholecystokinin (CCK) Phase 2 | N=19 | N=12 | |
| IRP (mmHg) | 123 (9, 155) | 6 (2, 13) | <0.001* |
| Esophageal Shortening (cm) | 5 (4, 6) | 4 (3, 5) | 0.01* |
| DCI (mmHg.cm.s) × 1000 | 30 (19, 49) | 8.6 (6, 12.9) | 0.001* |
| Relaxation (IRP<15 mmHg) | 5 (26%) | 12 (100%) | <0.001* |
| Atropine (ATR) | N=21 | N=18 | |
| LES Pressure (mmHg) | 21 (12, 30) | 16 (10, 20) | 0.1 |
| Wet Swallow IRP (mmHg) | 16 (13, 23) | 17 (13, 21) | 0.6 |
| Wet Swallow DCI (mmHg.cm.s) | 509 (236, 1185) | 233 (98, 626) | 0.08 |
Figure-1. Representative esophageal pressure topography plots following amyl nitrite and cholecystokinin administration in idiopathic (IA3) and opioid-induced (OA3) type three achalasia patients.
A) Note the dramatic rebound esophagogastric junction (EGJ) contraction observed in IA3 patients and its absence in OA3 patients. B) The middle IA3 example shows dramatic esophageal and EGJ contraction during both phases of the CCK response. OA3, on the other hand, shows an attenuated phase-1 CCK contractile response with absent phase-2 component. C) The bottom example shows that all phase-2 responses, when present, had a normal LES relaxations in OA3 patients. Note the greater phase-2 DCI in IA3 than OA3. IA3 showed normal phase-2 relaxation, which was observed in a small minority of these patients.
Cholecystokinin
All A3 patients showed motor responses starting 19 ± 8 seconds after CCK injection. The DCI of esophageal contractile response during phase-1 was significantly higher (p<0.0001) in IA3 than OA3 patients (Table 3). During the phase-1, 17/23 IA3 and 14/19 OA3 patients showed a paradoxical LES contractile response (p=1.0). However, the amplitude of the paradoxical contraction in IA3 patients was significantly higher (p<0.05) than OA3 patients. The phase-2 of esophageal response to CCK was seen in the majority of IA3 and OA3 patients. All 12/12 OA3 patients with presence of a phase-2 response, had an IRP less than 15 mmHg (Figure 1C), while 14 /19 of IA3 counterparts showed LES contraction averaging 123 mmHg (p<0.001, Figure 1B). The DCI of the terminating esophageal contractile response after phase-2 was significantly higher (p<0.001) in IA3 than OA3 patients (Table 3).
Atropine
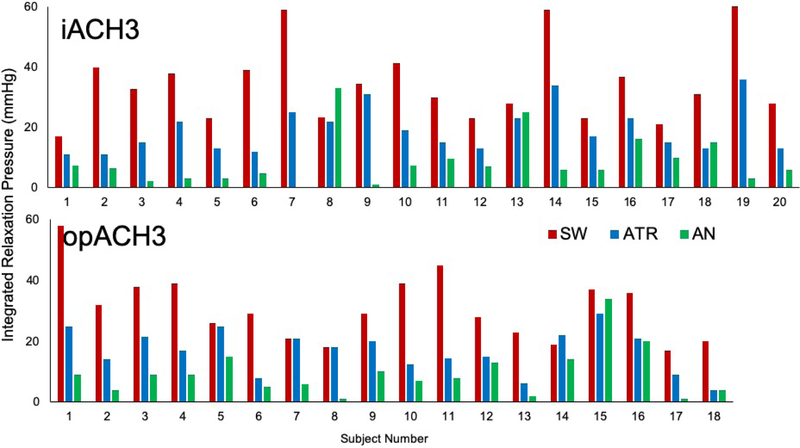
Cholinergic blockade using ATR caused a similar increase in heart rate in both groups. Atropine reduced the LES pressure, IRP and DCI in both IA3 and OA3 patients (p>0.05). While ATR reduced LES tone, this reduction was typically less than AN-induced relaxation (Figure-2). There was no difference between the two groups in cholinergic-sensitive portion of the LES tone (Table 3).
Figure-2. EGJ residual tone during wet swallow without pharmacologic agents (SW), wet swallow after atropine (ATR) and during amyl nitrite inhibitory response (AN).
Note the significant individual variability of atropine-sensitive presumably cholinergic-mediated portion of residual EGJ pressure.
Clinical Outcome
Clinical follow up data (24 ± 25 months) was available in 23 IA3 and 21 OA3 patients (Supplementary Tables). IA3 patients had significant improvement in dysphagia symptoms following both ablative and non-ablative therapy. The IA3 patients undergoing LES-ablative therapy had a higher prevalence of pain symptoms (p=0.01). Significantly more OA3 patients (91%) were managed by non-ablative therapy compared to 61% of IA3 patients (p=0.04). This included three patients who were completely weaned off of opioids (Figure 3). Similar to the IA3 patients, non-ablative therapy significantly improved dysphagia.
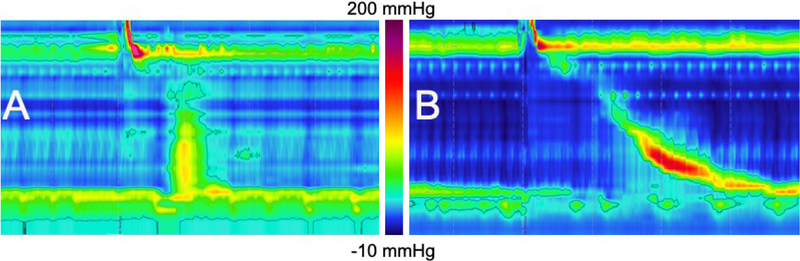
Figure 3: Reversible opioid-induced achalasia type 3 (OA3) following cessation of opioid therapy.
A) Initial EPT study is while patient is on daily opiate therapy, which shows findings of an A3 motor pattern. This patient’s pharmacologic response profile was typical of the opioid cohort (AN-induced rebound contraction= 28 mmHg; CCK phase-1 LES paradoxical contraction pressure = 97 mmHg; CCK phase-1 distal contractile integral (DCI) = 1464 mmHg.cm.s; CCK Phase-2 LES integrated relaxation pressure (IRP) =8 mmHg ) B) Repeat EPT study one year later, when patient was completely off opioid analgesics. There is now normal esophageal motility. Symptoms of solid and liquid dysphagia had resolved but chest pain persisted.
DISCUSSION
In this study we evaluated a cohort of patients whose manometric phenotype was consistent with type-3 achalasia. Nearly half of A3 patients were chronically on high doses of opioid analgesics (more than 60 milligram morphine equivalent daily dose), a condition known to be associated with substantial, but potentially reversible, alterations in esophageal motility. The idiopathic and opioid-induced achalasia patients could not be reliably differentiated on the basis of demographic features or clinical presentation. Likewise, their standard HRM parameters were similar. Both IA3 and OA3 patients show AN-induced relaxation gain and CCK-induced paradoxical contractile response that indicates impaired descending inhibition in both groups. Furthermore, ATR induced nearly 90% reduction in esophageal contractility (i.e. DCI) suggesting preservation of excitatory cholinergic signaling. However, despite similarities the two groups showed distinctive features in their responses to pharmacologic provocation.
AN Response:
Endogenous nitric oxide (NO) release from postganglionic inhibitory neurons in the myenteric plexus is necessary to achieve normal deglutitive LES relaxation12. AN is an inhaled organic nitrite and a potent exogenous NO donor. AN inhibits esophageal smooth muscle including the LES in both healthy controls and achalasia patients5. Near complete AN-induced EGJ relaxation in OA3 and IA3 patients indicate that impaired descending inhibition is the basis of outflow obstruction in both groups. In achalasia patients, termination of the AN-induced LES relaxation is typically associated with an exaggerated rebound phenomenon that is not seen in patients with normal esophageal motility8. The exact mechanism of this distinctive rebound contraction is unknown, but it may represent a form of denervation hypersensitivity in achalasia patients who have been chronically deprived of endogenous NO exposure8. What the OA3 patients do not seem to have is the distinctive rebound contraction seen in achalasia. The mechanism for this difference is unclear, but we speculate that this may be related to variable blockade of inhibitory neural pathways in OA3.
CCK Response:
CCK induces indirect LES relaxation through the activation of intramural inhibitory neurons, and direct excitation of smooth muscle fibers causing contraction of the LES6, 13. In healthy human subjects, physiologic postprandial endogenous CCK secretion14, 15 or lower pharmacologic doses of CCK11, 15 resulted in reduced LES tone. On the other hand in idiopathic achalasia patients, with presumed underlying ganglionic inhibitory dysfunction, pharmacologic doses of CCK usually resulted in a paradoxical LES contraction6. Recently, we described a biphasic CCK response in healthy human subjects that consisted of a phase-1 incomplete EGJ relaxation (preserved crural diaphragm contraction) followed by phase-2 complete EGJ relaxation (including the crural diaphragm inhibition)11. The phase-2 CCK response findings are phenotypically similar to the crural diaphragm inhibition and esophageal shortening observed during transient LES relaxations11, 16, 17. In the current study, we identified a similar biphasic response to CCK administration in 79 % of IA3 and 52% of OA3 patients. The phase-1 paradoxical LES contractile response in both the IA3 and OA3 confirms the abnormal inhibitory pathway in these patients. The paradoxical response in OA3 however, was significantly attenuated, as evidenced by a significantly lower LES contractile amplitude and esophageal DCI compared to IA3 patients (Table 3). In the IA3 patients exhibiting a phase-2 motor response, most IA3 patients (74%) had an abnormal contraction of the LES. This observation is similar to that of a previous report describing an aberrant TLESR response in achalasia patients18. In contrast, all OA3 patients with a phase-2 CCK response displayed LES relaxation (IRP < 15 mmHg). The preserved CCK phase-2 relaxation response in OA3 patients probably represents a pharmacologically driven response of the reversibly impaired inhibitory neurotransmission.
ATR Response:
In our study, ATR diminished the basal LES pressure and deglutitive DCI to a similar degree in both IA3 and OA3 patients. These findings are consistent with prior reports of preserved cholinergic innervation in achalasia patients5. However, the cholinergic mediated fraction of smooth muscle LES residual tone was highly variable across individual patients (Figure 2). Some patients had LES tone that was almost completely cholinergic mediated (based on an IRP after ATR that was equal to that seen with AN), although overall, IRP values after AN were significantly lower than after ATR.
Opioid-induced Peristalsis Disorder (OPD):
In healthy humans, morphine (100–200 mcg/kg) decreased the duration and amplitude of deglutitive LES relaxation19, 20. However, administration of naloxone did not significantly alter esophageal contractile response to deglutition or distention20. These findings suggested the potential for the development of esophageal dysmotility with pharmacologic doses of narcotics, although opioid receptors did not seem physiologically essential for normal peristalsis20. We have previously shown that, in chronic daily opioid users, opioid dose was significantly correlated with the degree of impairment of deglutitive LES relaxation (as evidenced by elevated IRP and IBP), consistent with blockade of normal inhibitory innervation3. Furthermore, in chronic opiate users the alteration in peristaltic sequence (reduced DL and increased CFV) and augmented contractile vigor (greater DCI) in the esophageal body suggested possible altered excitatory innervation3. Latter effects however, may be a compensatory esophageal response to the former EGJ outflow obstruction21. Attenuated esophageal contractile response to CCK may be a manifestation of preserved inhibitory neurotransmission reservoir that is propelled into action by pharmacologic provocation.
Relying on the patient’s reported opioid intake history may underestimate the prevalence and magnitude of opioid intake. Based on the national survey on drug use in the US, 49.2/1000 adults use prescription pain relievers for nonmedical purposes, and their source of obtaining opioids commonly cannot be identified by review of the medical records22. Case reports in the literature4 and our own clinical observation has been that OA3 patients have the potential for normalization of their motility pattern and symptomatic improvement with cessation/reduction of opioid therapy (Figure 3). Therefore, pharmacologic interrogation of A3 patients with AN and CCK may prove to be clinically useful in identifying those patients whose motor disturbance results from unrecognized opiate use and is thus potentially reversible. For example, based on our study findings, a patient with A3 phenotype in whom the AN-induced rebound contraction was < 38 mmHg (specificity=96%, PLR=9.4), or the CCK- induced phase-1 DCI was <1050 mmHg.cm.s (specificity=96%, PLR=10.1) would be highly likely to have OA3. On the other hand, an A3 patient with an AN-induced rebound contraction of > 125 mmHg (specificity=95%, PLR=9.4), or CCK-induced phase-1 paradoxical LES contraction of > 140 mmHg (specificity=95%, PLR=9.9), or CCK- induced phase-1 DCI > 12000 mmHg.cm.s (specificity=95%, PLR=9.9), or CCK-induced phase-2 IRP > 14 (specificity=95%, PLR=∞) or CCK- induced terminating after contraction DCI >27100 mmHg.cm.s (specificity=95%, PLR=∞) would likely have irreversible idiopathic destruction of the inhibitory neural pathways. Using any of the above diagnostic criteria, assuming a 50% pre-test probability, the post-test probability of predicted outcome surpasses 95%. Our predictive criteria, require independent validation in a larger prospective clinical trial.
Our study further supports the association of opioid use with major esophageal motor disorders. The differences in motor responses to pharmacologic challenge likely reflect the underlying differences in pathophysiology between idiopathic and opioid-induced esophageal motor disorders. The reports of reversibility of OPD are important to consider when contemplating invasive operative procedures for these patients. No intervention returns the patient to a state of normal motility, and all carry a risk of complications, such as a higher likelihood of reflux disease. Patients whose pharmacologic testing indicates a high likelihood of an OPD could be appropriately counselled about the potential benefits of opiate cessation on their esophageal symptoms.
In summary, in the current study we characterize the pharmacologic response of patients with type-3 achalasia motor pattern. Pharmacologic interrogation of OA3 patients shows preserved esophageal excitatory cholinergic innervation while confirming impairment of inhibitory innervation. Differences in the motor responses to pharmacologic challenges between IA3 and OA3 may prove useful clinically to differentiate these two patient groups and guide therapeutic decisions.
Supplementary Material
Supplementary Table 1. Clinical outcome of patients with idiopathic type 3 achalasia (IA3) treated by LES-ablative (N=9) versus non-ablative therapy (N=14). Categorical variables are shown as n (%). Pre and post values were compared using Fisher’s exact test. Only two patients were treated by pneumatic dilation in the LES ablative group without improvement in their esophageal symptoms. They were subsequently managed by botulinum toxin injection of esophagogastric junction (EGJ) and long acting calcium channel blockers. Three patients received endoscopic EGJ botulinum toxin injection and smooth muscle relaxants for their management in the non-ablative therapy group. All three reported symptom improvement during follow up.
Supplementary Table 2. Clinical outcome of patients with opioid-induced type-3 achalasia (OA3) treated by LES ablation (N=2) versus non-ablative medical therapy (N=19). Categorical variables are shown as n (%). Pre and post values were compared using Fisher’s exact test. Non-ablative therapy resulted in improved dysphagia but not regurgitation or pain. Both patients treated with LES-ablation had laparoscopic Heller myotomy.
WHAT YOU NEED TO KNOW.
Background
Some patients with achalasia type 3 (A3) use high doses of opioid analgesics. Manometry patterns in this group are indistinguishable from those of idiopathic A3 patients.
Findings
Nearly half of patients with an A3 pattern of dysmotility are chronic, daily users of opioids. Patients with opioid-induced A3 differ from patients with idiopathic A3 in responses to amyl nitrite and cholecystokinin.
Implications for patient care
These findings might be used to identify patients with dysmotility resulting from occult opiate use. Patients who appear to have opioid-induced achalasia could be counselled about the potential benefits of opioid therapy reduction for their esophageal symptoms.
Acknowledgments
This project was funded by institutional funds provided by the Department of Medicine at the Medical College of Wisconsin. In addition, it was in part supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001438; and by the Research and Education Program Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin. In addition, this project was funded by institutional funds provided by the Department of Medicine at the Medical College of Wisconsin.
GLOSSARY OF ABBREVIATIONS:
- OPD
OPIOID-INDUCED PERISTALTIC DISORDER
- LES
LOWER ESOPHAGEAL SPHINCTER
- EGJ
ESOPHAGOGASTRIC JUNCTION
- NO
NITRIC OXIDE
- AN
AMYL NITRITE
- TLESR
TRANSIENT LOWER ESOPHAGEAL SPHINCTER RELAXATION
- OA3
OPIOD INDUCED TYPE 3 ACHALASIA
- IA3
IDIOPATHIC TYPE 3 ACHALASIA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to disclose.
REFERENCES:
- 1.Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-Induced Esophageal Dysfunction (OIED) in Patients on Chronic Opioids. Am J Gastroenterol 2015;110:979–84. [DOI] [PubMed] [Google Scholar]
- 2.Ravi K, Murray JA, Geno DM, et al. Achalasia and chronic opiate use: innocent bystanders or associated conditions? Dis Esophagus 2016;29:15–21. [DOI] [PubMed] [Google Scholar]
- 3.Babaei A, Szabo A, Shad S, et al. Chronic daily opioid exposure is associated with dysphagia, esophageal outflow obstruction, and disordered peristalsis. Neurogastroenterol Motil 2019:e13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010;31:601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holloway RH, Dodds WJ, Helm JF, et al. Integrity of cholinergic innervation to the lower esophageal sphincter in achalasia. Gastroenterology 1986;90:924–9. [DOI] [PubMed] [Google Scholar]
- 6.Dodds WJ, Dent J, Hogan WJ, et al. Paradoxical lower esophageal sphincter contraction induced by cholecystokinin-octapeptide in patients with achalasia. Gastroenterology 1981;80:327–33. [PubMed] [Google Scholar]
- 7.Dodds WJ, Dent J, Hogan WJ, et al. Effect of atropine on esophageal motor function in humans. Am J Physiol 1981;240:G290–6. [DOI] [PubMed] [Google Scholar]
- 8.Babaei A, Shad S, Szabo A, et al. Pharmacologic interrogation of patients with esophagogastric junction outflow obstruction using amyl nitrite. Neurogastroenterol Motil 2019:e13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Given Imaging I. User Reference Guide, 2001–2013.
- 11.Babaei A, Mittal R. Cholecystokinin induces esophageal longitudinal muscle contraction and transient lower esophageal sphincter relaxation in healthy humans. Am J Physiol Gastrointest Liver Physiol 2018;315:G734–G742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tottrup A, Svane D, Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol 1991;260:G385–9. [DOI] [PubMed] [Google Scholar]
- 13.Behar J, Biancani P. Effect of cholecystokinin-octapeptide on lower esophageal sphincter. Gastroenterology 1977;73:57–61. [PubMed] [Google Scholar]
- 14.Zerbib F, Bruley Des Varannes S, Scarpignato C, et al. Endogenous cholecystokinin in postprandial lower esophageal sphincter function and fundic tone in humans. Am J Physiol 1998;275:G1266–73. [DOI] [PubMed] [Google Scholar]
- 15.Clave P, Gonzalez A, Moreno A, et al. Endogenous cholecystokinin enhances postprandial gastroesophageal reflux in humans through extrasphincteric receptors. Gastroenterology 1998;115:597–604. [DOI] [PubMed] [Google Scholar]
- 16.Pandolfino JE, Zhang QG, Ghosh SK, et al. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology 2006;131:1725–33. [DOI] [PubMed] [Google Scholar]
- 17.Babaei A, Bhargava V, Korsapati H, et al. A unique longitudinal muscle contraction pattern associated with transient lower esophageal sphincter relaxation. Gastroenterology 2008;134:1322–31. [DOI] [PubMed] [Google Scholar]
- 18.Kwiatek MA, Post J, Pandolfino JE, et al. Transient lower oesophageal sphincter relaxation in achalasia: everything but LOS relaxation. Neurogastroenterol Motil 2009;21:1294–e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowlatshahi K, Evander A, Walther B, et al. Influence of morphine on the distal oesophagus and the lower oesophageal sphincter--a manometric study. Gut 1985;26:802–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penagini R, Picone A, Bianchi PA. Effect of morphine and naloxone on motor response of the human esophagus to swallowing and distension. Am J Physiol 1996;271:G675–80. [DOI] [PubMed] [Google Scholar]
- 21.Mittal RK, Ren J, McCallum RW, et al. Modulation of feline esophageal contractions by bolus volume and outflow obstruction. Am J Physiol 1990;258:G208–15. [DOI] [PubMed] [Google Scholar]
- 22.Jones CM. Frequency of prescription pain reliever nonmedical use: 2002–2003 and 2009–2010. Arch Intern Med 2012;172:1265–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Clinical outcome of patients with idiopathic type 3 achalasia (IA3) treated by LES-ablative (N=9) versus non-ablative therapy (N=14). Categorical variables are shown as n (%). Pre and post values were compared using Fisher’s exact test. Only two patients were treated by pneumatic dilation in the LES ablative group without improvement in their esophageal symptoms. They were subsequently managed by botulinum toxin injection of esophagogastric junction (EGJ) and long acting calcium channel blockers. Three patients received endoscopic EGJ botulinum toxin injection and smooth muscle relaxants for their management in the non-ablative therapy group. All three reported symptom improvement during follow up.
Supplementary Table 2. Clinical outcome of patients with opioid-induced type-3 achalasia (OA3) treated by LES ablation (N=2) versus non-ablative medical therapy (N=19). Categorical variables are shown as n (%). Pre and post values were compared using Fisher’s exact test. Non-ablative therapy resulted in improved dysphagia but not regurgitation or pain. Both patients treated with LES-ablation had laparoscopic Heller myotomy.