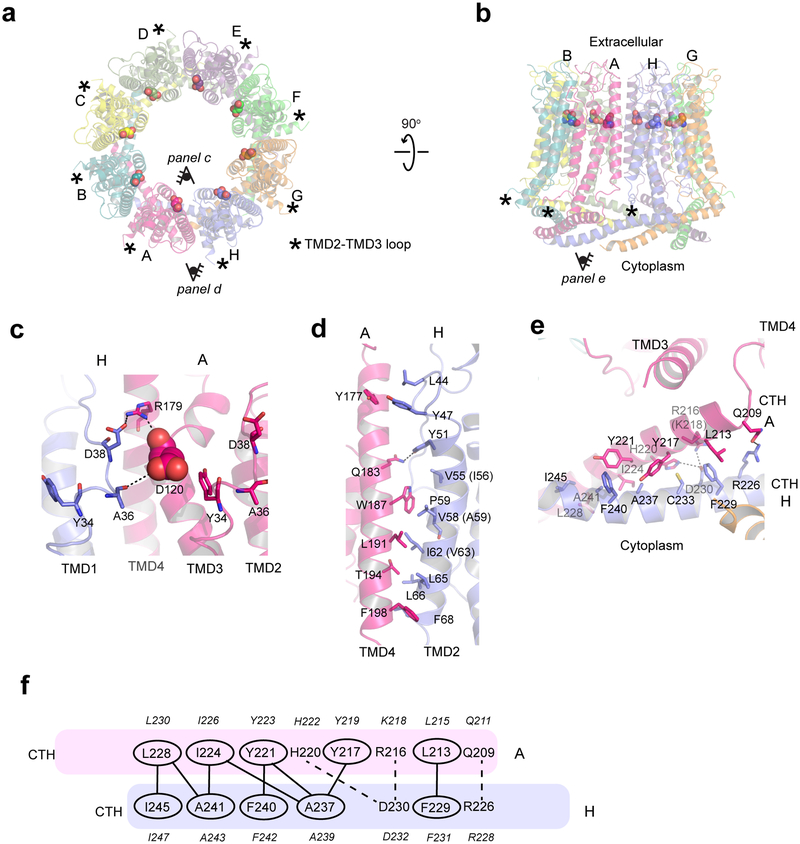
Fig. 2. Inter-subunit interface of chCALHM1.
a-b, The chCALHM1 structure viewed from the top of extracellular region (a) and the side of the membrane (b). Shown in spheres are the Asp120 residues critical for calcium sensitivity and ion permeation. c, Asp120 (sphere) and surrounding residues (sticks) form polar interactions to mediate inter-subunit interactions. d-e, The inter-subunit interactions between TMD2 and TMD4 (d) and CTHs (e). f, The schematic presentation of the interactions between two CTHs (magenta and slate blue). Polar and van der Waals interactions mediated by hydrophobic residues (ovals) are shown as dashed and solid lines, respectively. The residues in italic are the equivalent ones in hCALHM1.