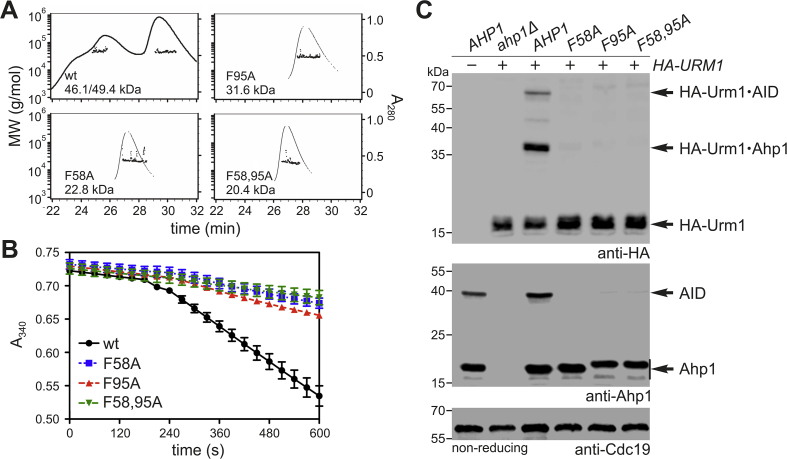
Fig. 5.
Ahp1 dimer interface mutations block peroxidase activity and urmylation. (A) Analysis of oligomeric state for Ahp1 variants by SEC-MALS. Traces represent A280 values of prominent peaks eluting off of the gel filtration column, and the scatter plotters underneath indicate the molecular weight (MW) ranges observed. The average molecular weight is given for the wild-type (wt) Ahp1 dimer and each interface mutant. (B) Coupled Ahp1 activity assays (see Materials & Methods). At 180 s, t-BOOH (100 μM) was added and NADPH absorbance at 340 nm monitored. An average of six independent measurements ± standard error of the mean is represented. (C) EMSA under non-reducing conditions from indicated strains expressing HA-URM1 (+) or not (−). NEM-stabilized urmylation was studied by anti-HA blot (top panel) diagnostic for free HA-Urm1 and urmylated forms of Ahp1 (~36 kDa) and Ahp1 intersubunit disulfides (AID ~72 kDa). anti-Ahp1 blot (middle panel) detects unmodified Ahp1 (~19 kDa) and AID (~38 kDa). Protein loading control: anti-Cdc19 (bottom panel).