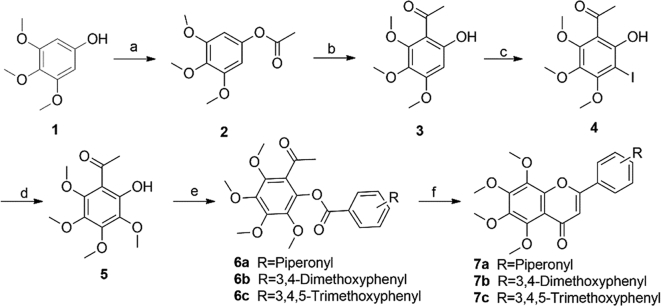
Scheme 1.
Syntheses of 5,6,7,8-tetramethoxy-2-phenyl-4H-chromen-4-ones 7a‒7c. Reagents and conditions: (a) Ac2O, AcOH, 110 °C, 2 h; (b) BF3·Et2O, AcOH, 70 °C, 2 h; (c) NIS, p-toluenesulfonic acid monohydrate, CH3CN, rt, 2 h; (d) 4 mol/L MeONa in MeOH, CuCl, DMF, 90 °C, 30 min; (e) acyl chlorides, Et3N, DCM, 0 °C to rt, 2 h; (f) triethylsilyl trifluoromethanesulfonate, Et3N, DCE, reflux, 2 h.