Highlights
-
•
Native aortic valve regurgitation (NAVR) presents technical challenges for TAVR.
-
•
This is the largest study on NAVR patients treated with the ACURATE neo valve.
-
•
Intraprocedural mortality was 0%, device success 87.5% and moderate PVL rate 8.3%.
-
•
Device success tended to be higher with perimeter-based >10% oversizing.
Abbreviations: AR, aortic regurgitation; AS, aortic stenosis; CHF, congestive heart failure; NAVR, native aortic valve regurgitation; NYHA, New York Heart Association; PVL, paravalvular leak; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve; VARC 2, Valve Academic Research Consortium 2
Keywords: Native aortic valve regurgitation, Transcatheter aortic valve replacement, Acurate Neo
Abstract
Background
Transcatheter aortic valve replacement (TAVR) has been validated for the treatment of severe symptomatic aortic stenosis in patients at high and intermediate surgical risk. Recently, TAVR has been proposed as an alternative to medical therapy in inoperable patients with severe native aortic valve regurgitation (NAVR). This multicenter international registry sought to evaluate safety and efficacy of TAVR with the self-expandable ACURATE neo valve in a cohort of patients with NAVR.
Methods
A total of 24 patients with severe NAVR treated by TAVR between September 2016 and October 2018 in 13 European centers were included. Clinical, procedural and follow up data were inserted in a dedicated database. Outcomes were codified according to Valve Academic Research Consortium-2 criteria.
Results
Mean age was 79.4 years, 58.4% were female. Mean EuroSCORE II and STS score were 5% and 3.9%, respectively. Device success was 87.5%. Moderate paravalvular leak (PVL) was found in two (8.3%) of patients, both with a perimeter oversizing index <10%. Implantation of a second device was necessary in three cases (12.5%), one for severe PVL and two for device displacement. New pacemaker implantation rate was 21.1%. At 30 days, stroke and all-cause mortality rates were 0% and 4.1%, respectively.
Conclusions
This multicenter study suggests good feasibility and early safety of transfemoral TAVR with the self-expandable ACURATE neo device in patients with severe NAVR refused for surgery. Rates of moderate PVL, new pacemaker implantation and need for a second valve were higher than those reported for TAVR in aortic stenosis.
1. Introduction
Transcatheter aortic valve replacement (TAVR) has become the standard of care for inoperable and high surgical risk patients affected by symptomatic severe aortic stenosis (AS), as well as an alternative to surgery for patients at low-to-intermediate risk [1], [2], [3], [4]. With over 500,000 procedures performed in more than 70 countries, TAVR has changed the paradigm in the treatment of AS [5], [6]. Growing operators’ experience and new transcatheter heart valve (THV) iterations have led to an expanded use of TAVR for the treatment of other valvular heart diseases when the patient is refused for surgery. Among these, native aortic valve regurgitation (NAVR) has always been considered a relative contraindication to TAVR because of the absence of calcification [7], [8], [9], [10], [11], [12], [13], which hinders THV anchoring and increases the risk of prosthesis embolization. However, some initial experiences of TAVR for NAVR have been reported, mainly with CoreValve™ (Medtronic, Ireland) and Edwards Sapien (Edwards Lifesciences, USA) systems [14], [15]. The self-expanding ACURATE neo valve (Boston Scientifics, USA) carries design features – such as the presence of stabilization arches – that may help to anchor the valve in the absence of calcifications as well as to ensure coaxial alignment, deployment stability and good sealing, but data on its use in the setting of NAVR are scant [16], [17]. Thus, this international multi-center registry aimed to evaluate the feasibility and early safety of TAVR with the ACURATE neo THV in a cohort of inoperable patients with NAVR.
2. Methods
2.1. Study design and patient population
The present study is an observational, non-randomized, multicenter registry including patients who underwent transfemoral TAVR with the ACURATE neo device. Inclusion criteria were as follows: (1) symptomatic severe NAVR (defined by transesophageal echocardiography according to current guidelines [1], with a mean transvalvular gradient <20 mmHg and a peak aortic jet velocity on continuous-wave Doppler of <2.5 m/s); (2) surgical aortic valve replacement refusal by the Heart Team; (3) anatomical features compatible with transfemoral ACURATE neo implantation (native annulus perimeter 66–85 mm, perimeter derived diameter 21–27 mm, absence of severe ascending aortic dilation [1]). Surgical risk was calculated using the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) and the Society of Thoracic Surgery (STS) score [18], [19]. Patients were informed about the off-label use of the ACURATE neo in NAVR. The registry was approved by the institutional review board of each institution according to the Declaration of Helsinki.
2.2. The device
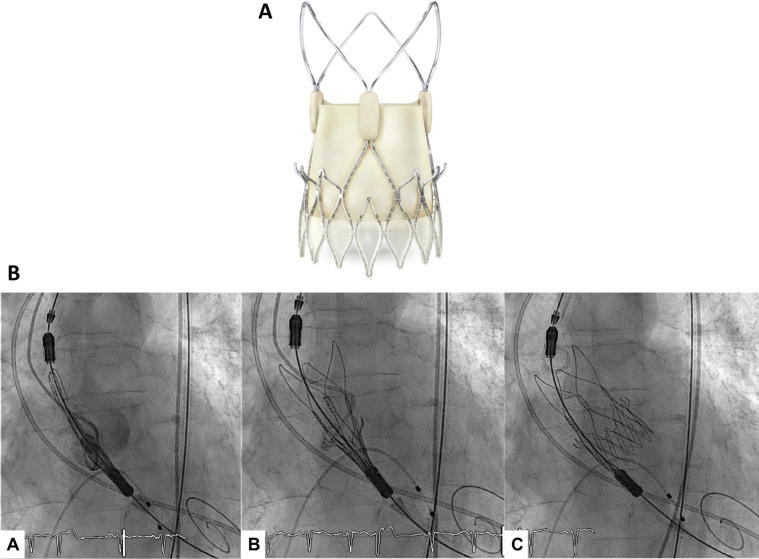
The ACURATE neo THV consists of a self-expandable nitinol stent frame with porcine leaflets in a supra-annular position (Fig. 1A). It is available in three sizes (23 mm, small; 25 mm, medium; 27 mm, large) covering an aortic annulus diameter between 21 and 27 mm. The stent frame is made of an upper and a lower crown, facilitating correct valve fixation in a sub-coronary and supra-annular position. The inflow part of the stent frame has a pericardial skirt to prevent paravalvular leakage (PVL). In the outflow part of the stent frame, three stabilization arches are used to align the bioprosthesis in the ascending aorta during deployment.
Fig. 1.
(A) The Accurate neo transfemoral prosthesis (from bostonscientifics.com). (B) Sequential angiographic images of the proximal end of stent holder into the left ventricular outflow tract by 5–7 mm (a), opening of the upper crown and stabilization arches (b), valve release (c).
2.3. TAVR procedure
All patients underwent multidetector computed tomography (CT-scan) to characterize aortic valve and aortic root anatomy. The total amount of calcifications was assessed semi-quantitatively and graded from none to severe as described elsewhere [20]. Procedures were performed under general anesthesia or conscious sedation according to local practice. The femoral access site was routinely pre-closed using Proglide or Prostar closure devices (Abbott Vascular, USA). The sequence of valve deployment is shown in Fig. 1B. Paravalvular leakage was assessed by aortography and echocardiography [21], [22], [23].
2.4. Endpoint definitions and clinical follow-up
Procedural outcomes and clinical events were adjudicated according to Valve Academic Research Consortium (VARC)-2 definitions [24]. The primary endpoint was device success defined by VARC-2 criteria. Secondary endpoints were all-cause and cardiovascular mortality rates at 30 days and 1 year, rehospitalization due to cardiac causes, post-procedural PVL at 30 days and other 30-day major clinical endpoints defined according to the VARC-2 criteria [24]. Follow-up was conducted through clinical visit or telephone interview and scheduled at 30-day and 1-year. The severity of PVL was assessed semi-quantitatively and graded using transthoracic echocardiography at each institution according to established criteria [1].
2.5. Statistical analysis
Data were analyzed with IBM SPSS® (version 24, SPSS, USA). The normality of the distribution for continuous variables was tested using the Shapiro-Wilk test. Continuous variables were presented as means ± standard deviation or median (minimum-maximum or interquartile range [IQR]), as appropriate. Categorical variables were presented as frequencies and percentages. Technical and procedural success rates were calculated as percentages of the total number of patients. Paired continuous parametric variables were compared using the paired Student’s t-test.
3. Results
3.1. Baseline characteristics
A total of 24 consecutive patients from 13 centres treated for severe NAVR with the ACURATE neo THV between September 2016 to October 2018 were included in the registry. Baseline characteristics are summarized in Table 1. The median age was 79.4 years (range 50–88), 14 patients were female (58.4%). Mean EuroSCORE II and STS were 5 ± 4.05% and 3.9 ± 2.37%, respectively. Contraindication to surgical aortic valve replacement was based on prohibitive surgical risk, as well as other factors such as previous cardiac surgery (12.5%), end-stage liver failure (4.1%), dialysis (4.1%) and/or combination of other comorbidities. Mean left ventricular ejection fraction was 48%, while mean end-diastolic diameter was 60 mm. At CT-scan, mean perimeter-derived diameter was 23.4 mm (range 20–26.3 mm), and approximately one-half patient had trivial or absent valve calcification (43.4%). Mean ascending aortic diameter was 34.5 mm (range 22–45.2). One patient (4.1%) had a bicuspid valve.
Table 1.
Baseline Characteristics.
| Clinical Characteristics | |
| Age, Yrs § | 79.4 (50–88) |
| Male | 10 (41.6%) |
| STS Score † | 3.9 ± 2.37 |
| Euroscore II † | 5 ± 4.05 |
| NYHA II NYHA III NYHA IV |
1 (4.1%) 22 (91.6%) 1 (4.1%) |
| GFR § | 59 (5–132) |
| Dialysis | 1 (4.1%) |
| Hypertension | 18 (75%) |
| Diabetes | 4 (16.6%) |
| Dislipemia | 11 (45.8%) |
| Chronic pulmonary Disease | 5 (20.8%) |
| End Stage Liver Failure | 1 (4.1%) |
| Prior CVA | 3 (12.5%) |
| Atrial fibrillation | 1 (4.1%) |
| Coronary artery disease | 6 (25%) |
| Prior PM | 5 (20.8%) |
| Prior MI | 0 (0.0%) |
| Prior PCI | 1 (4.1%) |
| Prior CABG | 1 (4.1%) |
| Prior Valve Surgery* | 2 (8.3%) |
| Transthoracic Echocardiography | |
| Left Ventricle Ejection Fraction, % § | 48.5 (30–65) |
| Left Ventricle End Diastolic Diameter, mm§ | 60 (41–83) |
| Mean Aortic Gradient, mmHg § | 13.8 (3–25) |
| Aortic Regurgitation 3+/4 4+/4 |
8 (33.3%) 16 (66.6%) |
| Mitral Regurgitation none 1+/4 2+/4 3–4+/4 |
3 (12.5%) 2 (8.3%) 6 (25%) 13 (54.1) |
| Pulmonary Hypertension | 7 (29.1%) |
| Ascending Aorta Diameter, mm § | 33.6 (22–45.2) |
| Multidetector Computed Tomography | |
| Annulus Area, mm2 § | 440 (299–510) |
| Annulus Perimeter, mm § | 77.1 (62.6–81.3) |
| Perimeter Derived Diameter, mm § | 23.4 (20–26.3) |
| Minimum Diameter, mm § | 20 (17.3–24.9) |
| Maximum Diameter, mm § | 26.1 (22.5–29.8) |
| Aortic valve calcifications Absent Mild Moderate Severe |
3 (12.5%) 8 (33.3%) 12 (50%) 1 (4.1%) |
| Ascending Aorta Diameter, mm § | 34.5 (22–45.2) |
| Bicuspid Valve | 1 (4.1%) |
STS = Society of Thoracic Surgeons; NYHA = New York Heart Association; CVA = cerebral vascular accident; PM = pacemaker; MI = myocardial infarction; CABG = coronary artery bypass graft; PCI = percutaneous coronary therapy. The GFR was calculated with the CKD-EPI formula.
Data are presented as mean ± standard deviation. § Data are expressed as median (min–max).
One patient underwent mitral valve surgery, the other pulmonary homograft surgery
3.2. Procedural data
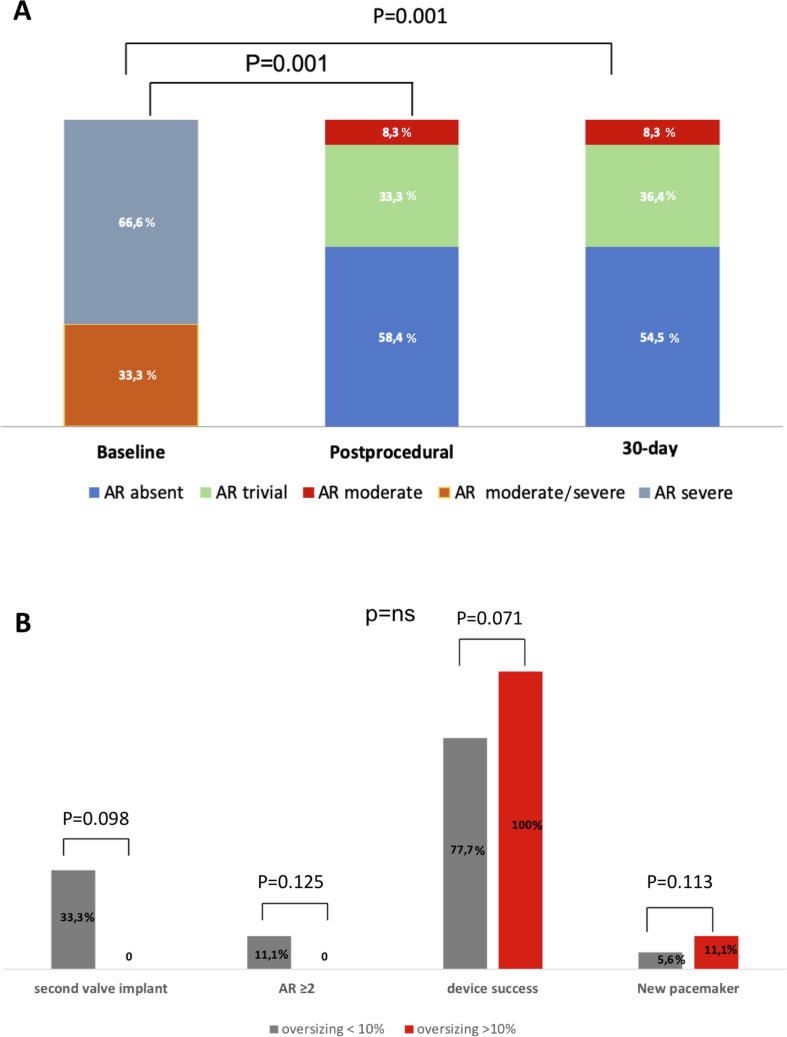
Procedural data are summarized in Table 2. According to VARC-2 definitions, device success was obtained in 21 patients (87.5%). Device failure was primarily due to implantation of a second device in 3 cases, in one case for severe PVL, in the other two because of displacement of the first THV. Notably, device migration occurred in patients with mild aortic calcifications. The second valve implanted was an Edwards Sapien 3 (Edwards Lifesciences, USA) in two cases and ACURATE neo in one case. Rapid pacing was used in 5 patients (23.8%). Post-dilation was performed in 4 patients (16.6%). Moderate PVL occurred in two cases (8.3%) (Fig. 2A). Intraprocedural mortality was 0%. Mean perimeter oversizing was 10.93% (5–20%). Both cases of valve migration were associated with a perimeter oversizing <10%. On the contrary, a perimeter oversizing >10% was associated with absent or trivial PVL in all cases (Fig. 2B).
Table 2.
Procedural data, short and mid-term outcomes.
| Procedural Characteristics | |
| Prosthesis Size: S M L |
1 (4.1%) 11 (45.9%) 12 (50%) |
| Oversizing, % | 10.9 (5–20%) |
| Procedure Time, min § | 59.9 (20–100) |
| Contrast Volume, ml § | 150 (30–330) |
| Fluoroscopy Time, min § | 19.9 (5–60) |
| Pre Implant BAV | 3 (12.5%) |
| Post Dilatation | 4 (16.6%) |
| Rapid Pacing | 5 (20.8%) |
| Successful Valve Deployment | 21 (87.5%) |
| Valve Migration | 2 (8.3%) |
| Implantation of a second valve | 3 (12.5%) |
| Conversion to Surgery | 0 (0.0%) |
| Coronary Obstruction | 0 (0.0%) |
| Cardiac Tamponade | 0 (0.0%) |
| Moderate/severe PVL | 1 (4.1%) |
| Device Success | 21 (87.5%) |
| In-hospital outcomes | |
| Death | 1 (4.1%) |
| Myocardial Infarction | 0 (0.0%) |
| Stroke (disabling and non-disabling) | 0 (0.0%) |
| Reintervention on aortic valve | 0 (0.0%) |
| Vascular Complications: Major Minor |
1 (4.1%) 3 (12.5%) |
| Bleeding: Major Minor |
2 (8.3%) 5 (20.8%) |
| New Pacemaker Implantation | 4 (21.1%) |
| Acute Kidney Injury | 0 (0%) |
| Moderate/severe PVL | 2 (8.3%) |
| NYHA Class I II III |
7 (29.1%) 15 (62.5%) 2 (8.3%) |
| 30-day follow up | |
| All-cause death | 1 (4.1%) |
| Cardiovascular death | 1 (4.1%) |
| Rehospitalization for CHF | 1 (4.1%) |
| NYHA Class I II III |
7 (30.4%) 15 (65.2%) 1 (4.3%) |
| Moderate/severe PVL | 2 (8.3%) |
| Aortic valve area, cm2 § | 1.9 (1.2–2.2) |
| Mean Aortic Gradient, mmHg § | 6.5 (4–16) |
| Early Safety | 22 (91.6%) |
| 1-year follow up (n = 17) | |
| All-cause death | 2 (11.7%) |
| Cardiovascular death | 2 (11.7%) |
| Rehospitalization for CHF Clinical Efficacy |
2 (11.7%) 15 (88.8%) |
† Data are presented as mean ± standard deviation. § Data are expressed as median (min–max).
BAV = balloon aortic valvuloplasty; PVL = paravalvular leakage; CHF = congestive heart failure
Early safety (VARC-2): combined endpoint at 30 days including all-cause mortality, all stroke, life-threatening bleeding, acute kidney injury stage 2 or 3, coronary artery obstruction requiring intervention, major vascular complication and valve-related dysfunction requiring repeat procedure.
Clinical efficacy (VARC-2): combined endpoint after 30 days including all-cause mortality, disabling or non-disabling stroke, or hospitalizations for valve-related symptoms or worsening congestive heart failure
Fig. 2.
(A) Change in aortic regurgitation from baseline to post-procedure up to 30-day follow up. (B) Rates of device success, post-procedural moderate aortic regurgitation, implantation of a second valve and new pacemaker implantation according to perimeter oversizing. AR = aortic regurgitation (includes both paravalvular leak and prosthetic valvular regurgitation).
3.3. Outcome data
In-hospital, 30-day and 1 year outcomes are depicted in Table 2. In-hospital mortality was 4.1%. In fact, one prohibitive surgical risk patient with severe left ventricular dysfunction died of heart failure. One patient experienced a major vascular complication (4.1%), two had a major bleeding (8.3%). The rate of new pacemaker implantation was numerically higher in patients with >10% oversizing (11.1% vs. 5.6%, p = 0.113). At discharge, 22 patients (91.6%) were in NYHA class I or II (p < 0.001 compared with baseline). Early safety was 91.6%. 30-day all-cause and cardiovascular mortality rate were 4.1%, with no case of stroke. 30-day echocardiographic follow-up showed a mean aortic valve area of 1.9 cm2 and a mean transvalvular gradient of 6.5 mmHg, with moderate PVL in 8.3% patients. One-year clinical follow-up was completed in 70.9% of patients. All-cause mortality was 8.3% with a median follow up time of 300 days (interquartile range: 6–843 days). One patient died after a major gastro-intestinal bleeding three months after TAVR, the other after a pacemaker lead endocarditis complicated with sepsis after four months from the procedure.
4. Discussion
The main findings of the current study, evaluating the largest available cohort of inoperable NAVR undergoing TAVR with the ACURATE neo THV, are as follows: (1) In this population of NAVR subjects refused for surgery, 30-day all-cause mortality was 4.1%, without any case of stroke; (2) Device success was 87.5%, the occurrence of moderate PVL was about 8% (with no case of severe PVL), while implantation of a second device was necessary in 12.5% of procedures; (3) Device success was achieved in all patients with a perimeter-based oversizing >10%, at the cost of higher new pacemaker implantation rates.
TAVR indications have been expanded not only to inoperable or high surgical risk patients, but also to intermediate and low-risk subjects with severe AS [1], [2], [5], [7], [8], [9], [10], [11], [25]. Pure NAVR has been considered a challenging scenario for THV implantation because of the frequent association with large aortic annulus and minimal aortic valve calcification [1]. Early experiences with first generation devices showed low rate of device success (79.1%) driven by high rates of second valve implantation and significant post-procedural PVL [26], [27]. Since then, the only approved valve system to be used in pure NAVR has been the transapical JenaValve™ (JenaValve Technology, Germany), with a reported device success rate of 96.5% [28], [29]. In the last few years, advancement of device technology and increasing operators’ experience led to an increased off-label use of TAVR in inoperable NAVR patients [14], [30], [31], [32]. In 2017 Yoon et al [15] published the largest registry of TAVR in patients with pure NAVR, reporting a device success rate as high as 81% with new generation THVs. In the latter study, ACURATE valve implantation rate was 1.5% and the ACURATE neo was not used at all.
In our study, the largest available on ACURATE neo valve for NAVR, THV implantation was feasible in all patients, and VARC-2 defined device success was 87.5%. This valve has design features that may help to anchor the valve even in the absence of calcification as well as to ensure coaxial alignment, deployment stability and good sealing, minimizing the risk of annular rupture and PM rate [14], [16], [29], [31], [33]. In our series, occurrence of moderate PVL was about 8% and implantation of a second THV was necessary because of device displacement or severe PVL in 3 patients (12.5%). These rates, although higher than those reported for the treatment of AS, are similar to those reported in NAVR series with different types of devices [15], [16], [17]. The 30-day and 1-year mortality rates were low (4.1% and 11.7%, respectively) and consistent with those reported in previous studies including patients with similar baseline surgical risk [7], [8], [9], [34]. Moreover, our findings compare favorably with those of other smaller series on the ACURATE neo for NAVR patients [16], [17].
In order to maximize TAVR device success in NAVR patients, particular attention should be paid during pre-procedural planning to device sizing. Several registries [20], [24], [35] and one meta-analysis [34] suggested that a relatively higher degree of device oversizing was associated with a reduction in post-procedural PVL rates, at the price of a higher PM rate. In our study, mean perimeter-based oversizing was 11% (range 5–25), slightly higher than previous experiences [17]. To note, an oversizing <10% was seen more often in patients post-procedural moderate PVL or implantation of a second valve. On the contrary, no patient with an oversizing >10% experienced any of these two complications. However, likely because of the low number of included patients, this association was not found to be statistically significant. Thus, larger studies are needed to confirm these findings. New pacemaker implantation rate was as high as 21.1%, almost double than that reported in other studies including patients with AS treated with the same device [32], [36], [37], but similar to other series dealing with NAVR[17]. Again, new pacemaker implantation was numerically higher in patients with an oversizing >10%. Importantly, a higher degree of oversizing did not result in any case of annular rupture, likely because of the low degree of aortic valve and left ventricular outflow tract calcification in our patients [33]. Finally, it is important to acknowledge that 1-year mortality and rehospitalization rates were higher than those of other TAVR studies on AS patients. This confirms previous findings reporting poorer outcome for inoperable NAVR patients as compared to AS subjects with similar risk profile [15], [28]. To this regard, we should note that most of our patients had moderate-severe left ventricular dilatation, over one third had reduced left ventricular ejection fraction and more than 50% had moderate or severe mitral regurgitation. This emphasizes the importance of adequate patient selection and optimal timing of TAVR in NAVR patients in order to improve overall survival.
5. Limitations
Limitations of this multicenter analysis include the relative low number of patients, as well as the lack of independent echocardiography core laboratory and event adjudication committee. Clinical and echocardiographic outcomes were self-reported, with inherent limitations and potential bias. Moreover, since the follow up was limited to one year, no inference about THV durability [38], [39] in the setting of NAVR can be made. Given the low rate of bicuspid aortic valve in our population, these results should not be generalized to patients with severe NAVR in the setting of bicuspid AS, for which TAVR is known to carry worse procedural outcomes [40], [41]. Moreover, ours represents a selected NAVR population mostly affected by organic aortic valve disease, and results should not be extended to functional NAVR in the setting of severe ascending aorta dilatation.
6. Conclusions
This preliminary experience on the largest available cohort of inoperable NAVR patients treated by transfemoral TAVR with the ACURATE neo THV suggests good feasibility and early safety of this approach. These results need to be confirmed by larger prospective studies in this population. Future device iterations should be aimed at reducing residual PVL, need for implantation of a second valve and pacemaker implantation in the setting of NAVR.
Declaration of Competing Interest
Ole de Backer: consultant for Abbott; Won-Keun Kim: received proctor fees from Symetis SA/Boston Scientific, St. Jude Medical/Abbott, lecture honoraria from Edwards Lifesciences, Symetis SA/Boston, St. Jude Medical/Abbott; Luis Nombela-Franco received proctor fees from Abbott, lecture honoraria from Edwards Lifesciences and Boston Scientific; Moritz Seiffert received lecture honorary or congress travel support from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic and Symetis. Fausto Castriota received proctor fes from Boston Scientific. Dr. Bedogni has served as a consultant for Abbott Vascular, Medtronic, Boston Scientific, and Terumo. Lars Sondergaard: received research grants and has been consultant for Abbott, Medtronic and Boston Scientific. Giuseppe Tarantini: proctor for Boston Scientific and received lecture fees from Edwards Lifesciences and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Baumgartner H., Falk V., Bax J.J., De Bonis M., Hamm C., Holm P.J. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 2.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3.Ueshima D., Fovino L.N., D'Amico G., Brener S.J., Esposito G., Tarantini G. Transcatheter versus surgical aortic valve replacement in low- and intermediate-risk patients: an updated systematic review and meta-analysis. Cardiovasc. Interv. Ther. 2019;34:216–225. doi: 10.1007/s12928-018-0546-5. [DOI] [PubMed] [Google Scholar]
- 4.Tarantini G., Nai Fovino L., D'Errigo P., Rosato S., Barbanti M., Tamburino C. Factors influencing the choice between transcatheter and surgical treatment of severe aortic stenosis in patients younger than 80 years: results from the OBSERVANT study. Catheter. Cardiovasc. Interv. 2019 doi: 10.1002/ccd.28447. [DOI] [PubMed] [Google Scholar]
- 5.Cribier A. Commemorating the 15-year anniversary of TAVI: insights into the early stages of development, from concept to human application, and perspectives. EuroIntervention. 2017;13:29–37. doi: 10.4244/EIJV13I1A3. [DOI] [PubMed] [Google Scholar]
- 6.Tarantini G., Nai Fovino L., Gersh B.J. Transcatheter aortic valve implantation in lower-risk patients: what is the perspective? Eur. Heart J. 2018;39:658–666. doi: 10.1093/eurheartj/ehx489. [DOI] [PubMed] [Google Scholar]
- 7.Adams D.H., Popma J.J., Reardon M.J., Yakubov S.J., Coselli J.S., Deeb G.M. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 8.Smith C.R., Leon M.B., Mack M.J., Miller D.C., Moses J.W., Svensson L.G. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 9.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 10.Reardon M.J., Van Mieghem N.M., Popma J.J., Kleiman N.S., Søndergaard L., Mumtaz M. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 11.Leon M.B., Smith C.R., Mack M.J., Makkar R.R., Svensson L.G., Kodali S.K. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 12.Dvir D., Webb J.G., Bleiziffer S., Pasic M., Waksman R., Kodali S. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–170. doi: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
- 13.Yoon S.H., Bleiziffer S., De Backer O., Delgado V., Arai T., Ziegelmueller J. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J. Am. Coll. Cardiol. 2017;69:2579–2589. doi: 10.1016/j.jacc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Roy D.A., Schaefer U., Guetta V., Hildick-Smith D., Möllmann H., Dumonteil N. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J. Am. Coll. Cardiol. 2013;61:1577–1584. doi: 10.1016/j.jacc.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Yoon S.H., Schmidt T., Bleiziffer S., Schofer N., Fiorina C., Munoz-Garcia A.J. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J. Am. Coll. Cardiol. 2017;70:2752–2763. doi: 10.1016/j.jacc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Wendt D., Kahlert P., Pasa S., El-Chilali K., Al-Rashid F., Tsagakis K. Transapical transcatheter aortic valve for severe aortic regurgitation: expanding the limits. JACC Cardiovasc. Interv. 2014;7:1159–1167. doi: 10.1016/j.jcin.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Toggweiler S., Cerillo A.G., Kim W.K., Biaggi P., Lloyd C., Hilker M. Transfemoral implantation of the acurate neo for the treatment of aortic regurgitation. J. Invasive Cardiol. 2018;30:329–333. [PubMed] [Google Scholar]
- 18.Saffioti S., Burzotta F., Coluccia V., Trani C., Bruno P., Massetti M. Usefulness of EuroSCORE systems for risk stratification. J. Cardiovasc. Med. (Hagerstown) 2015;16:90–99. doi: 10.2459/JCM.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 19.Shahian D.M., He X., Jacobs J.P., Rankin J.S., Welke K.F., Filardo G. The society of thoracic surgeons isolated Aortic Valve Replacement (AVR) composite score: a report of the STS quality measurement task force. Ann. Thorac. Surg. 2012;94:2166–2171. doi: 10.1016/j.athoracsur.2012.08.120. [DOI] [PubMed] [Google Scholar]
- 20.John D., Buellesfeld L., Yuecel S., Mueller R., Latsios G., Beucher H. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc. Interv. 2010;3:233–243. doi: 10.1016/j.jcin.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Barbanti M., Yang T.H., Rodès Cabau J., Tamburino C., Wood D.A., Jilaihawi H. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation. 2013;128:244–253. doi: 10.1161/CIRCULATIONAHA.113.002947. [DOI] [PubMed] [Google Scholar]
- 22.Nai Fovino L., Badawy M.R.A., Fraccaro C., D'Onofrio A., Purita P.A.M., Frigo A.C. Transfemoral aortic valve implantation with new-generation devices: the repositionable Lotus vs. the balloon-expandable Edwards Sapien 3 valve. J. Cardiovasc. Med. (Hagerstown) 2018;19:655–663. doi: 10.2459/JCM.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 23.Fovino L.N., Saladini G., Mormino G.P., Saladini F., Razzolini R., Evangelista L. Risk stratification and prognostic assessment by myocardial perfusion-gated SPECT in patients with left bundle-branch block and low-intermediate cardiac risk. Ann. Nucl. Med. 2012;26:559–570. doi: 10.1007/s12149-012-0613-4. [DOI] [PubMed] [Google Scholar]
- 24.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 25.Ueshima D., Barioli A., Nai Fovino L., D'Amico G., Fabris T., Brener S.J. The impact of pre-existing peripheral artery disease on transcatheter aortic valve implantation outcomes: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2019 doi: 10.1002/ccd.28335. [DOI] [PubMed] [Google Scholar]
- 26.Testa L., Latib A., Rossi M.L., De Marco F., De Carlo M., Fiorina C. CoreValve implantation for severe aortic regurgitation: a multicentre registry. EuroIntervention. 2014;10:739–745. doi: 10.4244/EIJV10I6A127. [DOI] [PubMed] [Google Scholar]
- 27.Vahanian A., Alfieri O., Andreotti F., Antunes M.J., Barón-Esquivias G., Baumgartner H. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur. J. Cardiothorac. Surg. 2012;42:S1–S44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 28.Seiffert M., Diemert P., Koschyk D., Schirmer J., Conradi L., Schnabel R. Transapical implantation of a second-generation transcatheter heart valve in patients with noncalcified aortic regurgitation. JACC Cardiovasc. Interv. 2013;6:590–597. doi: 10.1016/j.jcin.2013.01.138. [DOI] [PubMed] [Google Scholar]
- 29.Seiffert M., Bader R., Kappert U., Rastan A., Krapf S., Bleiziffer S. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc. Interv. 2014;7:1168–1174. doi: 10.1016/j.jcin.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Schofer J., Nietlispach F., Bijuklic K., Colombo A., Gatto F., De Marco F. Transfemoral implantation of a fully repositionable and retrievable transcatheter valve for noncalcified pure aortic regurgitation. JACC Cardiovasc. Interv. 2015;8:1842–1849. doi: 10.1016/j.jcin.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Urena M., Himbert D., Ohlmann P., Capretti G., Goublaire C., Kindo M. Transcatheter aortic valve replacement to treat pure aortic regurgitation on noncalcified native valves. J. Am. Coll. Cardiol. 2016;68:1705–1706. doi: 10.1016/j.jacc.2016.07.746. [DOI] [PubMed] [Google Scholar]
- 32.Kim W.K., Hengstenberg C., Hilker M., Kerber S., Schäfer U., Rudolph T. The SAVI-TF Registry: 1-year outcomes of the european post-market registry using the ACURATE neo transcatheter heart valve under real-world conditions in 1,000 patients. JACC Cardiovasc. Interv. 2018;11:1368–1374. doi: 10.1016/j.jcin.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Tarantini G., Basso C., Fovino L.N., Fraccaro C., Thiene G., Rizzo S. Left ventricular outflow tract rupture during transcatheter aortic valve implantation: anatomic evidence of the vulnerable area. Cardiovasc. Pathol. 2017;29:7–10. doi: 10.1016/j.carpath.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Yousef A., MacDonald Z., Simard T., Russo J.J., Feder J., Froeschl M.V. Transcatheter Aortic Valve Implantation (TAVI) for native aortic valve regurgitation – a systematic review. Circ. J. 2018;82:895–902. doi: 10.1253/circj.CJ-17-0672. [DOI] [PubMed] [Google Scholar]
- 35.Blanke P., Pibarot P., Hahn R., Weissman N., Kodali S., Thourani V. Computed tomography-based oversizing degrees and incidence of paravalvular regurgitation of a new generation transcatheter heart valve. JACC Cardiovasc. Interv. 2017;10:810–820. doi: 10.1016/j.jcin.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Ueshima D., Nai Fovino L., Mojoli M., Napodano M., Fraccaro C., Tarantini G. The interplay between permanent pacemaker implantation and mortality in patients treated by transcatheter aortic valve implantation: a systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2018;92:E159–E167. doi: 10.1002/ccd.27681. [DOI] [PubMed] [Google Scholar]
- 37.Möllmann H., Hengstenberg C., Hilker M., Kerber S., Schäfer U., Rudolph T. Real-world experience using the ACURATE neo prosthesis: 30-day outcomes of 1,000 patients enrolled in the SAVI TF registry. EuroIntervention. 2018;13:e1764–e1770. doi: 10.4244/EIJ-D-17-00628. [DOI] [PubMed] [Google Scholar]
- 38.Tarantini G., Purita P.A.M., D'Onofrio A., Fraccaro C., Frigo A.C., D'Amico G. Long-term outcomes and prosthesis performance after transcatheter aortic valve replacement: results of self-expandable and balloon-expandable transcatheter heart valves. Ann. Cardiothorac. Surg. 2017;6:473–483. doi: 10.21037/acs.2017.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nai Fovino L., Scotti A., Massussi M., Fabris T., Cardaioli F., Rodinò G. Incidence and feasibility of coronary access after transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2020 doi: 10.1002/ccd.28720. [DOI] [PubMed] [Google Scholar]
- 40.Tarantini G., Fabris T., Cardaioli F., Nai Fovino L. Coronary access after transcatheter aortic valve replacement in patients with bicuspid aortic valve: lights and shades. JACC Cardiovasc. Interv. 2019 doi: 10.1016/j.jcin.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 41.Ueshima D., Nai Fovino L., Brener S.J., Fabris T., Scotti A., Barioli A. Transcatheter aortic valve replacement for bicuspid aortic valve stenosis with first- and new-generation bioprostheses: A systematic review and meta-analysis. Int. J. Cardiol. 2019 doi: 10.1016/j.ijcard.2019.09.003. [DOI] [PubMed] [Google Scholar]