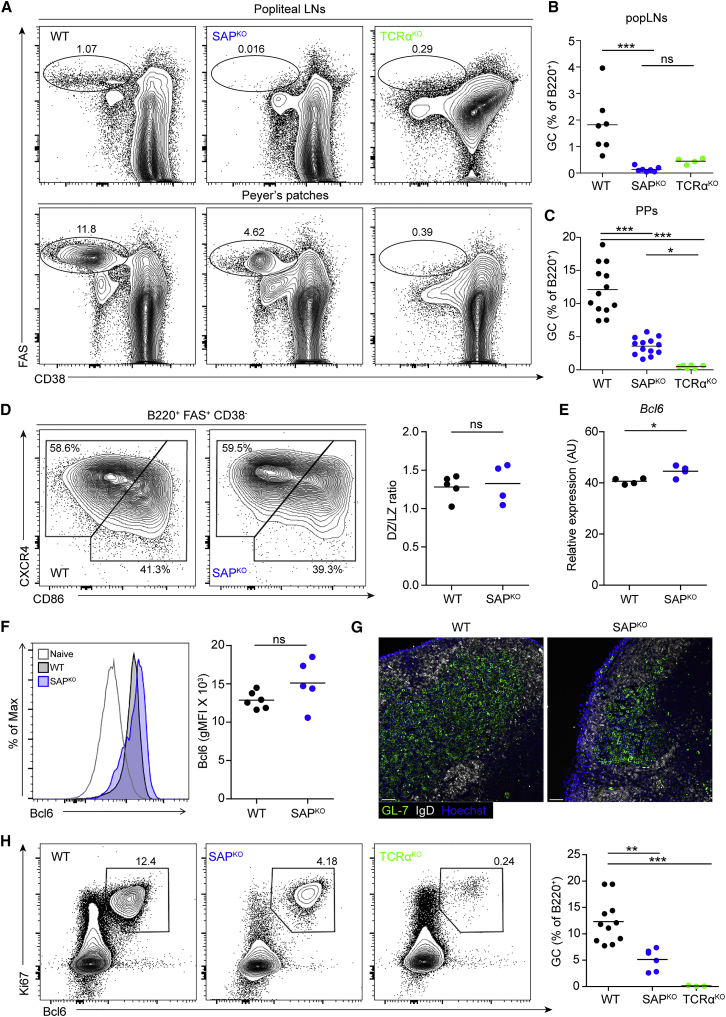
Figure 2.
GC B Cells in PPs of SAP-Deficient Mice Express Typical Markers
(A) Representative flow cytometry plots showing GC B cells (CD38− FAS+) in popliteal LNs (top panel) 7 days after intra-footpad NP-OVA immunization and in PPs of WT, SAPKO, or TCRαKO mice.
(B and C) Quantification of GC frequency in popliteal LNs (B) and PPs (C), as in (A).
(D) Representative flow cytometry plots and graph of dark zone (DZ) (CXCR4hi CD86lo) and light zone (LZ) (CXCR4lo CD86hi) GC B cell distribution in the PPs of WT and SAPKO mice.
(E) Bcl6 transcript in sorted GC B cells derived from PPs of WT and SAPKO mice.
(F) Representative histogram and quantification of Bcl6 expression in GC B cells of WT and SAPKO mice; the naive B cell population is shown as a negative control.
(G) Representative images of mediastinal LNs of influenza-infected mice. LNs were fixed, sectioned, and stained for GL-7 (fluorescein isothiocyanate [FITC], green) and IgD (AF-647, white) to mark the GC and the B cell follicle. Hoechst was used for nuclear staining. Scale bar, 50 μm.
(H) Representative flow cytometry plots showing GC B cells (Ki67+ Bcl6+) in the mediastinal LNs of WT, SAPKO, and TCRαKO mice, 14 days following influenza infection. GC frequencies are summarized in the graph.
Data are pooled from two (B, D–F, and H) and four (C) independent experiments. Each dot represents a single mouse; line represents the mean. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.0001, one-way ANOVA with Bonferroni posttest in (B), (C), and (H) and two-tailed Student’s t test in (D)–(F). ns, not significant.