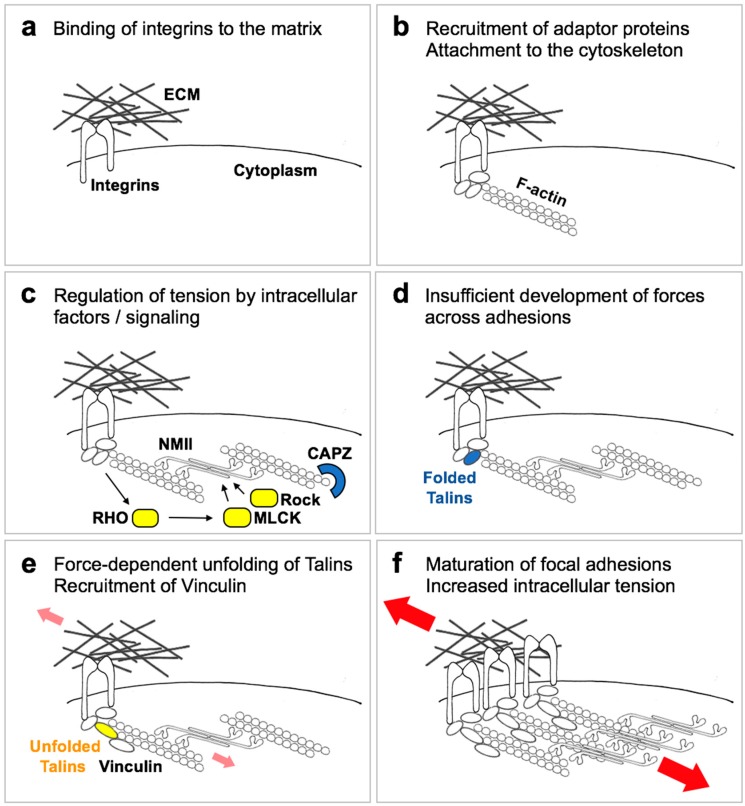
Figure 2.
A simplified scheme of ECM mechanosensing. When cells adhere to an ECM via integrin receptors, cell-substrate adhesions are transiently formed (a). These adhesions are stabilized by recruitment of adaptor proteins to integrin cytoplasmic domains, which in turn connect to actin filaments (F-actin), leading to formation of focal points (b). Locally activated signaling proteins (c) regulate the development of myosin (NMII)-mediated tension, counteracted by negative regulators such as CAPZ. If cell-generated tension is not opposed by extracellular resistance (d), such as on a soft ECM, adhesions remain at this stage or disassemble. If instead a stiff ECM efficiently opposes resisting forces (small red arrows), tension across adhesion complexes induce the unfolding of adaptor proteins such as Talin1/2 and the recruitment of new players, including Vinculin (e). This enables the growth and reinforcement of cell-substrate adhesions that mature into focal adhesions (f), in turn sustaining the development of higher cell traction forces (large red arrows).