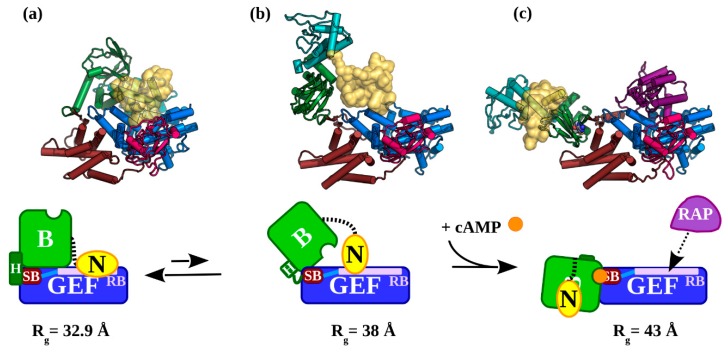
Figure 6.
The conformational states adopted by EPAC1 during cAMP-induced activation: (a) Apo-EPAC1 in its inactive (EPAC1closed) state. The NTD, marked N (yellow), interacts with the catalytic GEF domain to block the effector binding site: RB (mauve). The Hinge (green helix), is fully formed and the switchboard (SB, brown) is held away from the cAMP binding site. The HP connecting the SB to the RB is shown in light blue. (b) The inactive (EPAC1extended) is observed with approximately a 13% frequency. This fraction of EPAC1 is receptive to activation by cAMP binding, because the Hinge has melted and the SB’s lid has not yet closed over the open cAMP binding site in the CNBD of “B”. (c) The active, cAMP-bound (EPAC1open) state of EPAC1 is capable of binding effectors such as Rap1b (purple) to the RB. The observed Rg for each state is given under each model. The atomic models are coloured as in Figure 1. The EPAC1 cartoons are coloured by region: N: NTD (yellow); regulatory B: DEP and CNBD (green); and the catalytic GEF: the REM, RA, and GEF domains (blue). In brown is the SB region, the Hinge (H) helix is dark green, and the RB region is mauve.