Figure 1.
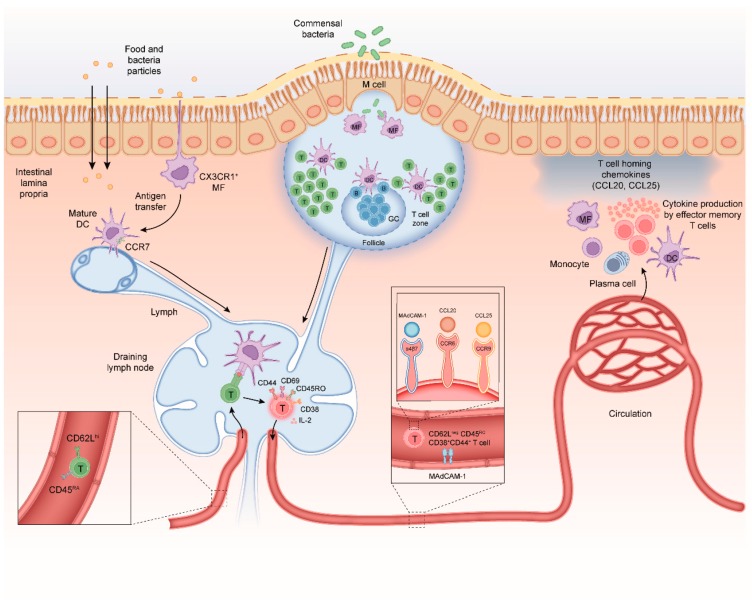
Mechanisms of CD4+ T-cell differentiation in the healthy intestine. (i) Antigens can be taken up by specialized microfold cells (M-cells) that can transport microbial antigens across the mucosal epithelium from the lumen to the subepithelial dome in the Peyer’s patch (PP); (ii) soluble antigens can diffuse through epithelial tight junctions and can be transferred across epithelial cells by transcellular routes and be ingested by phagocytes such as macrophages and dendritic cells (DC); and (iii) luminal antigens can also be captured by transepithelial projecting dendrites from macrophage-like CX3CR1+ APCs in the lamina propria. M-cells and CX3CR1+ APCs can transfer antigen to migratory dendritic cells that migrate from the lamina propria to inductive sites to interact with naïve CD4+ T-cells. Naïve CD4+ CD45RA chemokine receptor C-C motif receptor 7+ (CCR7) T-cells migrate from the peripheral blood into gut-draining lymph nodes and PP. The DC-mediated T-cell activation requires three signals; (i) T-cell receptor (TCR) stimulation with antigenic peptides in the context of major histocompatibility complex class II (MHCII), (ii) co-stimulation via CD28-CD80/CD86 engagement and (iii) cytokine signals mostly provided by APCs. Upon activation, T-cells acquire CD69, CD25 (IL-2 receptor-alpha chain) CD44 and CD45RO. T-cells down-regulate expression of CD62L, CCR7, and CD45RA and start secreting IL-2, a cytokine needed for proliferation. After a 3–4-day proliferation period the differentiated T-cells egress from the lymph node into the blood. A subpopulation of these CD45ROCD44+CD4+ T-cells will re-express CD62Lhi and CCR7 and provide central memory in lymphoid tissues. The remainder of the CD62LnegCD45ROCD44+CD4+ T-cells will migrate to the intestinal lamina propria where they reside as long-lived memory CD4+ T-cells. Crucially, differentiation of T-cells in the MLN is associated with imprinting of higher levels of retinoic acid-induced CD38. As this CD62LnegCD38+ effector T-cell phenotype is induced regardless of regulatory or inflammatory T-cell function it can be used to monitor gut-homing T-cells in human peripheral blood. Homing of memory-phenotype CD62LloCD45ROCD44+CD4+ T-cells to the small and large intestine requires expression of the integrin α4β7 which binds to mucosal vascular addressin cell adhesion molecule 1 (MADCAM1) on endothelial cells in blood vessels. In the small intestine, epithelial cells produce the chemokine CCL25 which attracts memory T-cells expressing the receptor CCR9 Chemokine receptors involved in regulation of CD4+ T-cell homing to the colon at steady state are less well defined, although recently, it was discovered that G protein coupled receptor 15 (GPR15) directs CD4+ T-cells to the colon. During intestinal inflammation, CCR6 and its ligand CCL20 also contribute to CD4+ T-cell recruitment to the inflamed small and large intestine, demonstrating that CD4+ T-cells can use alternative homing receptors during inflammation when compared to steady state.