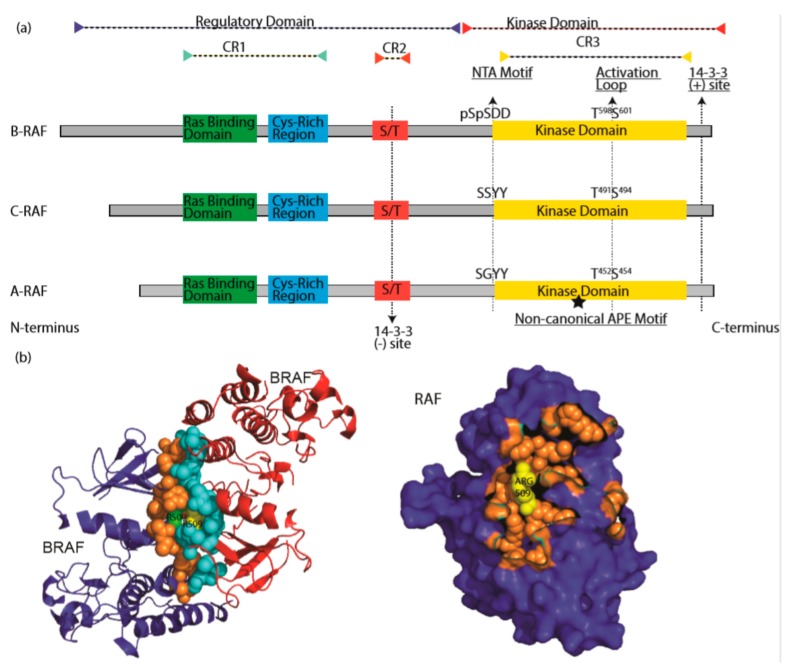
Figure 2.
The RAF family kinases. (a) Conserved domains on three RAF proteins are shown in panel (a). CR1 contains a Ras binding domain and a Cys-rich region while CR2 includes a S/T phosphorylation site. The 14-3-3 binding at this region inhibits RAF. CR3 contains a putative kinase domain adjacent to an acidic N-terminus (NTA) and a regulatory C-terminus. At the C terminus, there is a secondary 14-3-3 binding site which promotes dimerization. The non-canonical APE motif of ARAF is labeled with a star. ((b), left) Dimer interface of BRAF is shown, crystallography data was obtained from [86], PDB ID: 4MNE. Blue and red color indicates two separate BRAF molecules. Orange (Blue BRAF) and turquoise colored (Red BRAF) sphere-shaped amino acids indicate the dimer interface. While R509 from both RAF molecule located at the center of the dimer interface, green R509 belongs to red BRAF, while yellow R509 does to blue BRAF. ((b), right) Crystal structure of dimer interface from plane of interaction with only blue BRAF chain is visible. Orange spheres indicate the amino acids at the dimer interface, with exception of Arg509, which is labeled with yellow color. Structure was drawn by using pyMOL. CR1: conserved region 1; CR2: conserved region 2; CR3: conserved region 3.