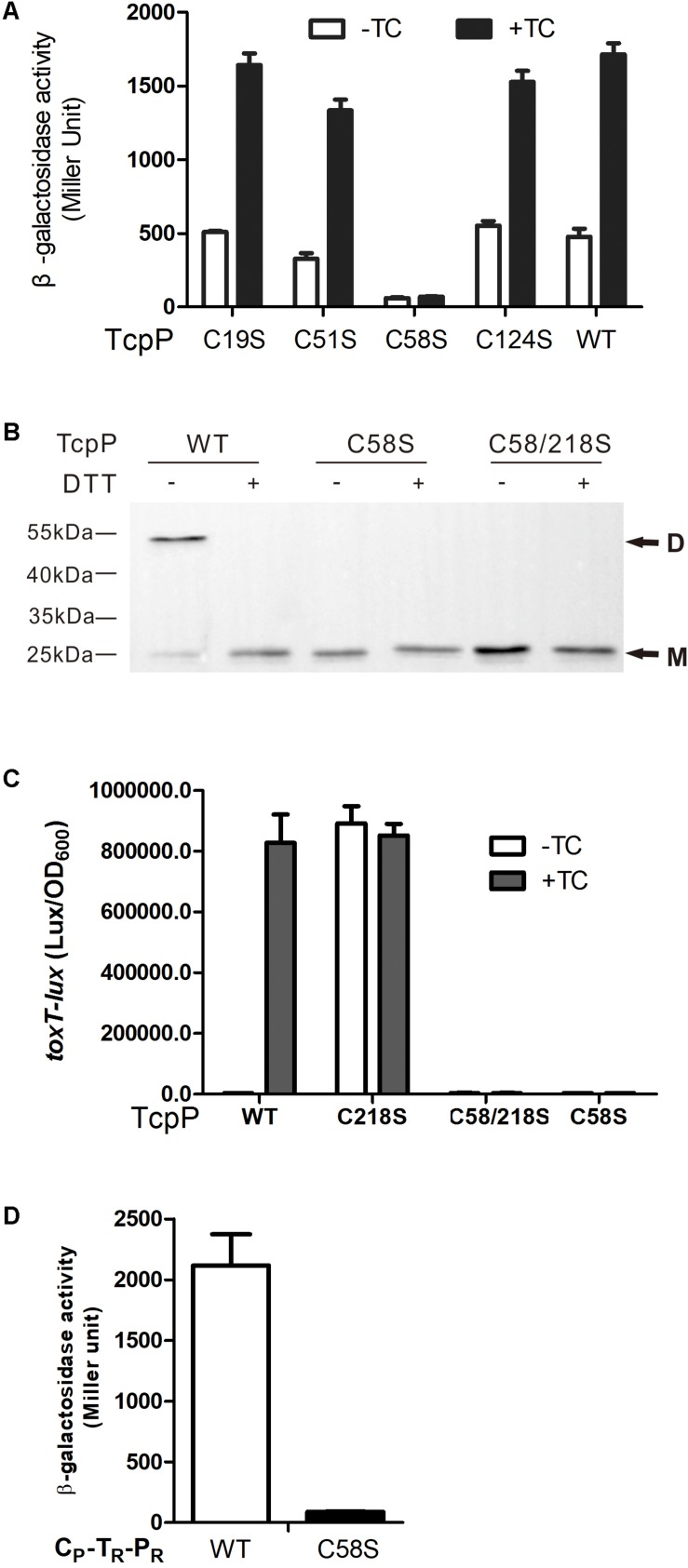
FIGURE 3.
C58 of TcpP is indispensable for protein dimerization. (A) Full-length TcpP wild type or its cysteine mutant was fused with the T25 and T18 domains of adenylate cyclase (CyaA) from Bordetella pertussis, respectively, and the T25, T18 fusion pairs were introduced into E. coli cyaA mutants (Karimova et al., 1998). Cultures were grown at 30°C for 8 h without shaking and β-galactosidase activity was measured and reported as Miller Units (Miller, 1972). Data are means ± SD (n = 3). (B) V. cholerae ΔtcpP containing PBAD-controlled plasmids harboring TcpP or its cysteine mutant derivatives fused with N-terminal FLAG tags were grown in LB containing 0.01% arabinose in the presence of taurocholate acid at 37°C for 6 h. Cell lysates (0.1 mg) were applied to SDS-PAGE with (+) or without (–) 50 mM of DTT, and subjected to Western blotting using an anti-FLAG antibody. D, dimer; M, monomer. (C) E. coli DH5a strains containing PBAD-controlled plasmids harboring TcpP or its cysteine mutant derivatives and a PtoxT-lux transcriptional fusion plasmid were grown in LB with 0.01% arabinose in the presence or absence of taurocholate acid at 37°C until OD600 ≈ 0.2. Luminescence was measured and reported as light units/OD600. Data are the means ± SD (n = 3). (D) Chimeric TcpP was fused with the T25 and T18 domains and the T25, T18 fusion pairs were introduced into E. coli cyaA mutants (Karimova et al., 1998). Cultures were grown at 30°C for 8 h without shaking and β-galactosidase activity was measured and reported as Miller Units (Miller, 1972). Data are means ± SD (n = 3).