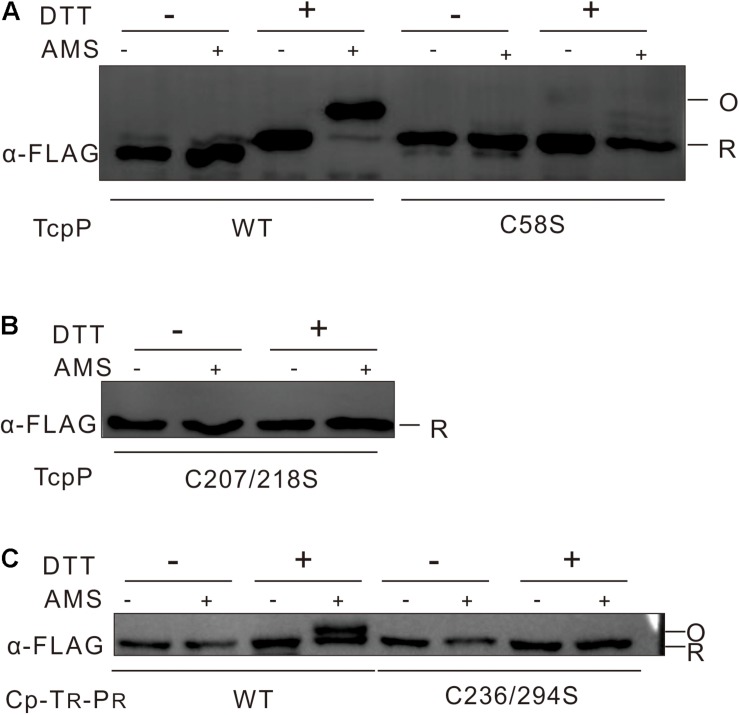
FIGURE 4.
No disulfide bond could be detected in TcpP cytoplasmic domain. (A) V. cholerae ΔtcpP containing PBAD-controlled plasmids harboring TcpP or its cysteine mutant derivatives fused with C-terminal FLAG tags were incubated at 37°C until OD600 ≈ 0.5. To label free thiol groups irreversibly, 100 mM iodoacetamide was added directly to the living cells. After TCA precipitation and extensive washing, oxidized thiol groups were reduced by addition of 50 mM DTT (+) in denaturing buffer or not (–). These reduced cysteines were then alkylated by addition of 10 mM AMS (+) or not (–). Samples were mixed with non-reducing SDS-sample buffer and loaded onto 12.5% SDS-polyacrylamide gels. TcpP was detected by Western blot analysis of the FLAG-tagged proteins. Blot shown is representative of at least three separate experiments. (B) Disulfide bond assay of V. cholerae ΔtcpP strain containing PBAD-controlled plasmids harboring TcpPC207/218S was used the same methods as described in panel A. (C) Disulfide bond assay of E. coli strain containing chimeric TcpP was used the same methods as described in panel A.