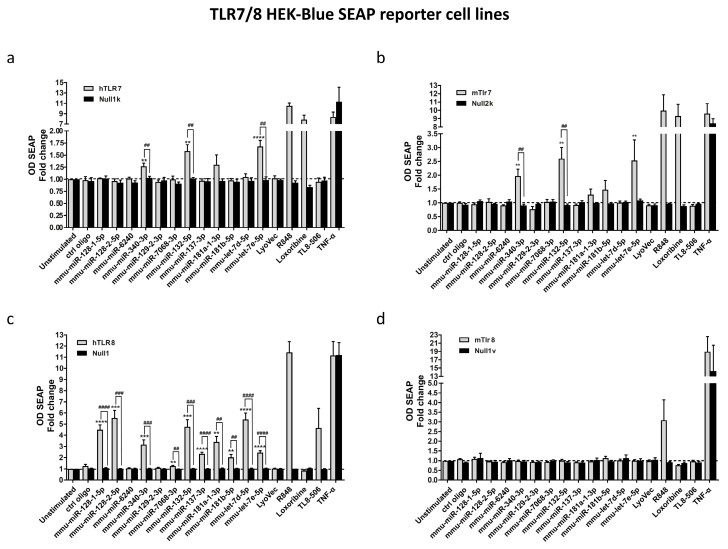
Figure 2.
Extracellularly delivered miRNAs activate TLR7/8 in HEK293-derived TLR reporter cells. Human (a,c) or murine (b,d) TLR7 or TLR8 HEK-Blue-secreted embryonic alkaline phosphatase (SEAP) reporter cells and the corresponding null control cell lines were incubated with 10 µg/mL of the indicated synthetic oligoribonucleotides identified from small RNA sequencing analysis of supernatants from apoptotic neurons for 24 h. LyoVec and unstimulated cells served as negative controls, while loxoribine (TLR7), resiquimode (R848; TLR7/8) and TL8-506 (TLR8) served as positive control for the respective TLR reporter. Mutant oligoribonucleotide (ctrl oligo, 10 µg/mL) was used as a negative control. TNF was applied to activate the NF-κB/AP-1 promoter in reporter and control cells. SEAP protein was detected at an optical density (OD) of 655 nm and depicted as fold change of the SEAP protein normalized to unstimulated control. (a) Response of human TLR7 HEK-Blue reporter and control cells (Null1k) to indicated miRNAs. (b) Response of murine TLR7 HEK-Blue reporter and control cells (Null2k) to indicated miRNAs. (c) Response of human TLR8 HEK-Blue reporter and control cells (Null1) to indicated miRNAs. (d) Response of murine TLR8 HEK-Blue reporter and control cells (Null1v) to indicated miRNAs. Depicted are n = 4–6 individual experiments. Significances were obtained by Student’s t-Test. (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; * compared to unstimulated control; # compared to HEK Null parental control cells). Data are represented as mean ± s.e.m.