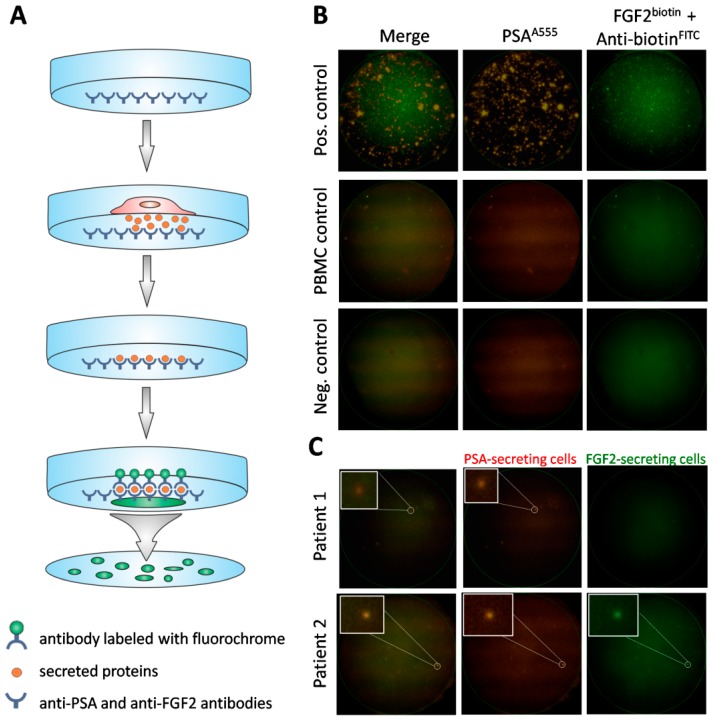
Figure 2.
Detection of viable CTCs using the dual fluorescent EPISPOTPSA/FGF2 assay. (A) Procedure of the assay. Membrane covered with anti-PSA and anti-FGF2 antibodies, culture of cells enriched via depletion of CD45+ cells, binding of secreted proteins to previously immobilized antibodies, immunostaining of secreted proteins caught on the membrane. (B) Positive and negative controls. Culture of LNCaP (shown in figure) and NBTII cells (secreting PSA and FGF2, respectively) as positive controls, culture of PBMC (not secreting PSA nor FGF2) and wells without cells used as negative controls. A single immunospot corresponds to the protein “fingerprint” of one viable cell. (C) Patient samples. Representative examples of CTCs defined based on PSA-secretion (Patient 1) or PSA and additionally, FGF2 (Patient 2). PSA-positive, FGF2-positive and double PSA/FGF2 immunospots (merge) correspond to viable CTCs. Immunospots were detected and observed using the C.T.L. Elispot Reader, 50× magnification.