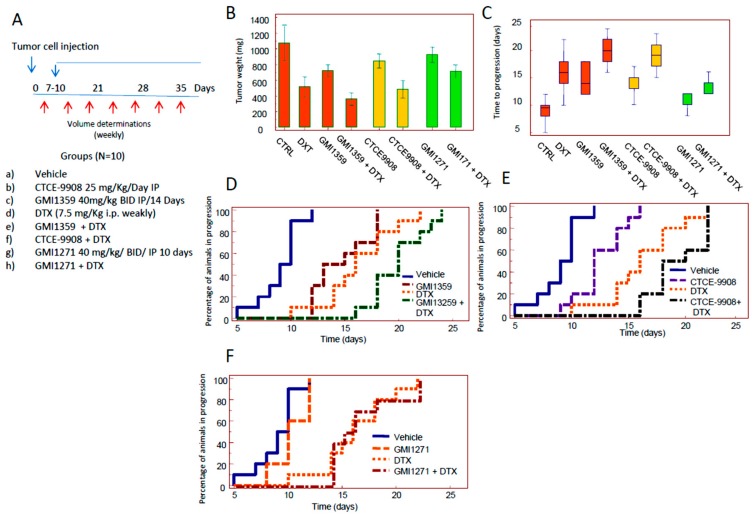
Figure 5.
In vivo experiments: comparison of the DTX sensitizing effects of GMI-1359, GMI-1271 and CTCE-9908 administered in subcutaneous 22rv1 xenograft model. (A) Diagram of treatments: tumour bearing animals were randomized approximately 10 days or when tumors reached 80–100 mm3 of volume in eight experimental groups as follows: Group 1: mice receiving intraperitoneal (i.p.) injections of 100 µL PBS (vehicle); Group 2: mice receiving CTCE-9908 (25 mg/kg, i.p); Group 3: mice receiving GMI-1359 twice a day ip at 40 mg/ kg for consecutive 14 days; Group 4: mice received DTX (7.5 mg/Kg/week; Group 5: mice receiving GMI-1359 and DTX at doses described above for single administration; Group 6. mice receiving CTCE-9908 and DTX at doses described above for single administrations; group 7: mice receiving GMI-1271 40 mg/Kg once daily; Group 8: mice receiving GMI-1271 and DTX at doses described above for single administrations.(B) Tumor weight distribution in our treatment cohort. (C) TTP analysis. (D–F) Kaplan Meyer representation for the analyses of chemo-sensitizing effects of GMI-1359 (D), CTCE-9908 (E) and GMI-1271 (F) in 22rv1 xenograft model. Statistics for the comparisons of hazard ratios are shown in Supplementary Materials Figure S1C.