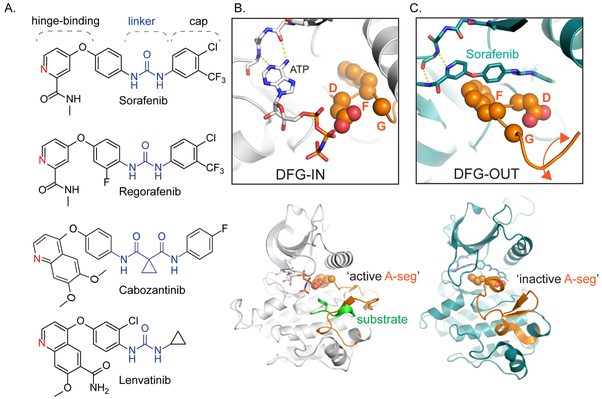
Figure 1: Clinical kinase inhibitors for HCC share common structural features that enable conformation specific binding.
A. Sorafenib and regorafenib are both approved for first-line and second-line treatment of HCC; their chemical structures differ by a single fluorine. Cabozantinib and lenvatinib are also promising therapies for HCC. All four compounds share several conserved structural motifs: the hinge-binding element functions as an adenosine mimic, the linker and cap extend deep into the kinase active site pocket. B. Top: Phosphorylated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog within an active state DFG (aspartate-phenylalanine-glycine)-IN configuration (PDB ID: 1IR3), where the ATP analog is able to interact with the DFG loop, and thus amenable to both substrate recognition and phosphorylation (Bottom). C. Top: Binding of sorafenib to BRAF (PDB ID: 1UWH) forces the kinase to adopt the DFG-OUT conformation. In this state, the BRAF activation-segment (A-seg; bottom) adopts an inactive conformation. The DFG-OUT form of binding is referred to as Type II inhibition.