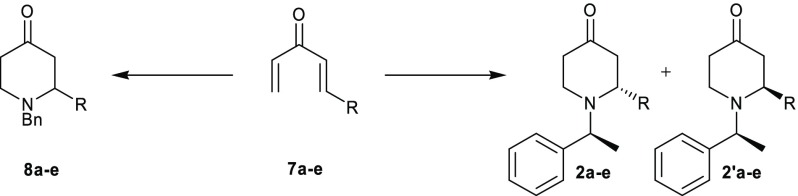
Table 2. Synthesis of 2-Substituted Racemic and Diastereomeric 4-Piperidones.
| entry | R | 8 (%)a | 2 + 2′ (%)b | ratiod of 2:2′ |
|---|---|---|---|---|
| 1 | Me | a (42)c | a (37)c | 1.1:1.0 |
| 2 | n-Pr | b (36)c | b (27)c | 1.4:1.0 |
| 3 | Ph | c (79) | c (63) | 2.6:1.0 |
| 4 | 4-ClC6H4 | d (84) | d (68) | 2.8:1.0 |
| 5 | 4-OMeC6H4 | e (84) | e (57) | 3.7:1.0 |
Reaction conditions: 7a–e, benzylamine, NaHCO3, CH3CN/H2O (3:1), 16 °C (40 min) → 95 °C, 1.5 h.
Reaction conditions: 7a–e, S-α-phenylethylamine, NaHCO3, CH3CN/H2O (3:1), 16 °C (40 min) → 95 °C, 1.5 h.
Crude ketone used as starting material.
The ratio was derived from the isolated product yields (for entries 1, 4, and 5) or determined by analysis of the 1H NMR spectra (for entries 2 and 3).