Abstract
Studies on living turtles have demonstrated that shells are involved in the resistance to hypoxia during apnea via bone acidosis buffering; a process which is complemented with cutaneous respiration, transpharyngeal and cloacal gas exchanges in the soft-shell turtles. Bone acidosis buffering during apnea has also been identified in crocodylian osteoderms, which are also known to employ heat transfer when basking. Although diverse, many of these functions rely on one common trait: the vascularization of the dermal shield. Here, we test whether the above ecophysiological functions played an adaptive role in the evolutionary transitions between land and aquatic environments in both Pseudosuchia and Testudinata. To do so, we measured the bone porosity as a proxy for vascular density in a set of dermal plates before performing phylogenetic comparative analyses. For both lineages, the dermal plate porosity obviously varies depending on the animal lifestyle, but these variations prove to be highly driven by phylogenetic relationships. We argue that the complexity of multi-functional roles of the post-cranial dermal skeleton in both Pseudosuchia and Testudinata probably is the reason for a lack of obvious physiological signal, and we discuss the role of the dermal shield vascularization in the evolution of these groups.
This article is part of the theme issue ‘Vertebrate palaeophysiology’.
Keywords: acidosis buffering, cutaneous respiration, heat transfer, historical constraints
1. Introduction
The vertebrate post-cranial dermal skeleton is composed of bony scutes which ossify within the dermis [1–4]. The presence of these bony elements varies taxonomically, and the resulting shield morphology results from both the shape and the relative position of the dermal plates. These bones can be juxtaposed or articulated as observed in stem archosaurs [5,6], in pseudosuchians [7–9] and some squamates [10]; they can also be fused as in turtles [11,12] and xenarthrans [13,14] or be isolated as in some ornithischian and sauropod dinosaurs [15–18].
Continuous shields of osteoderms (e.g. Aetosauria, Xenarthra) or bony scutes (e.g. Testudinata) have mostly been considered for their protective aspects against predators [19–23]. However, experimental investigations on turtles have shown that their dermal shield would also have ecophysiological functions. Indeed, the bone tissues composing the shield would be able to buffer the acidosis which is caused by blood pH decrease after both the blood CO2 pressure has increased and the lactic acid (lactate) has been produced via fermentation during prolonged apnea [24–28]. Bone acidosis buffering consists of supplying mineral elements such as (1) bicarbonates that can bind to the free protons which are dissolved in the blood plasma (due to respiratory acidosis) and (2) calcium that can complex with the lactate and thus inhibits its acidity (in answer to metabolic acidosis) [29].
Such a physiological process has also been identified in the osteoderms of crocodylians [30] which are known to be semi-aquatic animals, derived from terrestrial ancestors [5,8,31]. In addition, crocodylian osteoderms are also involved in heat transfer with the environment during emerged and semi-emerged basking periods [17,23,32] via the enclosed vessels of which blood flow is controlled by cardiac activity and vasomotion, thus regulating the distribution of heat to the vital organs [33–35]. Even though no specific studies have yet been performed on this aspect in the testudinatans, their dermal shield must also be involved in heat transfer [36], since it covers the majority of the body surface while enclosing peripheral blood vessels within bone cavities [37,38].
We quantified the post-cranial dermal bone vascular area as a proxy to assess the number and size of the blood vessels that are both enclosed within bony cavities and closely in contact with the apical bone surface when a superficial ornamentation is present. Indeed, the sculptural elements that compose the bone ornamentation are known to provide vascular openings contributing to the dermal plate global vascularization by conducting blood vessels to the overlying soft dermis [39] as observed in pseudosuchians [40], tryonichids [37] and helochelydrids [41,42]. We then analysed the data with phylogenetic comparative methods (in Pseudosuchia and Testudinata) to reveal whether the post-cranial dermal bone vessel proliferation is: (1) influenced by the phylogeny and (2) correlated with lifestyle transitions unrelated to the phylogenetic relationships.
2. Material and methods
(a). Sampling strategy
We studied 31 cross sections of dermal bones coming from different parts of the shell of both extant and extinct testudinatan species (dry bones and well-preserved fossils) from museum collections or published articles (table 1). The cross sections are transverse and pass by the centre of the sampled bones. The taxonomic affiliation and lifestyle attributes of the fossil forms could be identified unambiguously based on anatomical features. We classified the specimens into three categories depending on their lifestyle: terrestrial, freshwater and marine. When there was no ambiguity regarding the taphonomy (post-mortem transportation), the nature of the sediment was also used as a clue to infer their living environment (e.g. marine versus fresh water; electronic supplementary material, S1).
Table 1.
Description of the sample, (a) Testudinata and (b) Pseudouchia. TMM: Texas Memorial Museum (Austin USA); FMNH: Field Museum of Natural History (Chicago, USA); MCNA: Museo de Ciencias Naturales de Alava (Vitoria-Gasteiz, Spain); YPM: Yale Peabody Museum (New Haven, USA); UPUAM: Unidad de Paleontología, Universidad Autónoma de Madrid (Spain); WU-SILS-RH: Waseda University (Tokyo, Japan); NSMT: National Museum for Nature and Science of Tokyo (Japan); ZIN PH: Zoological Institute (Russian Academy of Sciences, Saint Petersburg); FPDM: Fukui Prefectural Dinosaur Museum (Katsuyama City, Fukui Prefecture, Japan); UA: Université d'Antananarivo (Madagascar); SMNS: Smithsonian Institution; BSPG: Bayerische Staatssammlung für Paläontologie und Geologie, München, Germany; PEFO: Petrified Forest National Park, USA; ISI: Indian Statistical Institute (Calcutta, India); UCMP: University of California, Museum of Paleontology (Berkeley, USA); MNHN: Muséum National d'Histoire Naturelle; IPB: Institute of Paleontology (Bonn, Germany); NMS: Naturmuseum Solothurn, Switzerland; MCL: Musée des confluences (Lyon, France); PVL: Colección de Paleovertebrados del Instituto Miguel Lillo (Tucumán, Argentina); n.a.: non-attributed.
| porosity | lifestyle | region | ornamentation | age | collection number | |
|---|---|---|---|---|---|---|
| (a) Testudinata | ||||||
| Hesperotestudo sp. | 0.07 | terrestrial | flat osteoderm | no | Pleistocene | TMM 30967-1010.1 |
| Hesperotestudo sp. | 0.21 | terrestrial | spiked osteoderm | no | Pleistocene | TMM 30967-1010.2 |
| Terrapene carolina tringuis | 0.40 | terrestrial | neural | no | extant | FMNH 211806 |
| Terrapene carolina tringuis | 0.22 | terrestrial | costal (right) | no | extant | FMNH 211806 |
| Dorkota vasconica | 0.21 | freshwater | costal | no | Barremian | MCNA 14366 |
| Dorkota vasconica | 0.24 | freshwater | neural | no | Barremian | MCNA 14372 |
| Podocnemis erythrocephala | 0.15 | freshwater | sample costal | no | extant | YPM 11853 |
| Solemys sp. | 0.07 | terrestrial | costal fragment | yes | Maastrichtyian | UPUAM-14001 |
| Solemys vermiculata | 0.14 | terrestrial | costal fragment | yes | Maastrichtyian | MCNA 15047 |
| Solemys vermiculata | 0.16 | terrestrial | shell fragment | yes | Maastrichtyian | MCNA 15046 |
| Carettochelys insculpta | 0.10 | freshwater | costal (right 7th) | yes | extant | WU-SILS RH1044 |
| Pelodiscus sinensis | 0.11 | freshwater | costal | yes | extant | NSMT-H 6600 |
| Trionychidae indet. | 0.11 | freshwater | costal | yes | Aptian-Albian | ZIN PH 102 |
| Trionychidae indet. | 0.10 | freshwater | costal | yes | early Cenomanian | ZIN PH 122 |
| Trionychidae indet. | 0.09 | freshwater | costal | yes | Barremian–Aptian | FPDM V0127 |
| Bothremys barberi | 0.31 | marine | costal | no | Campanian | FM P27406 (FMNH) |
| Bothremys barberi | 0.32 | marine | costal | no | Campanian | FM P27406 (FMNH) |
| Bothremys barberi | 0.30 | marine | neural | no | Campanian | FM P27406 (FMNH) |
| Caretta caretta | 0.39 | marine | costal | no | extant | FMNH 98963 |
| Caretta caretta | 0.33 | marine | hyoplastron | no | extant | FMNH 98963 |
| Archelon ischyros | 0.26 | marine | shell fragment | no | Late Cretaceous | YPM 1783 |
| Plesiochelys sp. | 0.18 | marine | neural | no | Kimmeridgian | NMS 8730 |
| Taphrosphys sulcatus | 0.34 | marine | costal | no | Maastrichtian | YPM 40288 |
| Taphrosphys sulcatus | 0.35 | marine | neural | no | Maastrichtian | YPM 40288 |
| Ctenochelys stenoporus | 0.36 | marine | neural | no | Campanian | FM PR 442 |
| Geochelone elegans | 0.14 | terrestrial | costal | no | extant | IPB 561-C |
| Geochelone elegans | 0.10 | terrestrial | costal | no | extant | IPB 561-C |
| Geochelone elegans | 0.07 | terrestrial | neural | no | extant | IPB 561-C |
| Hesperotestudo crassiscuta | 0.19 | terrestrial | neural | no | Pleistocene | ROM 5540 |
| Hesperotestudo crassiscuta | 0.30 | terrestrial | plaston fragment | no | Pleistocene | ROM 5541 |
| Hesperotestudo crassiscuta | 0.28 | terrestrial | shell fragment | no | Pleistocene | ROM 5542 |
| (b) Pseudosuchia | ||||||
| Araripesuchus tsangatsangana | 0.05 | terrestrial | n.a. | yes | Late Cretaceous | UA 9966 |
| Batrachotomus kupferzellensis | 0.01 | terrestrial | paramedian pre-caudal | yes | Late Ladinian | SMNS 80317 |
| Prestosuchus chiniquensis | 0.05 | terrestrial | sacral paramedian | yes | Late Ladinian/Early Carnian | BSPG ASXXV7 |
| ‘Prestosuchus’ loricatus | 0.04 | terrestrial | pre-caudal paramedian | yes | Late Ladinian/Early Carnian | BSPG ASXXV46d |
| Rauisuchus tiradentes | 0.16 | terrestrial | pre-caudal paramedian | yes | Late Carnian/Early Norian | BSPG ASXXV121b |
| Revueltosaurus sp. | 0.04 | terrestrial | paramedian | yes | Norian | PEFO 33787 |
| Tikisuchus romeri | 0.14 | terrestrial | pre-caudal paramedian | yes | Carnian | ISI R 305/ 1 |
| Simosuchus clarki | 0.1 | terrestrial | n.a. | no | Late Cretaceous | UA 9965 |
| Simosuchus clarki | 0.07 | terrestrial | n.a. | no | Late Cretaceous | UA 9965 |
| Yarasuchus deccanensis (Avemetatarsalia) | 0.10 | terrestrial | pre-caudal paramedian | yes | Anisian | ISI R 334 |
| Alligator mississippiensis | 0.15 | semi-aquatic | n.a. | yes | extant | SMNS 10481b |
| Allognathosuchus wartheni | 0.13 | semi-aquatic | n.a. | yes | Wasatchian | UCMP 113731 |
| Crocodylus niloticus | 0.13 | semi-aquatic | dorsal | yes | extant | MNHN-AC- 1920.90, PC |
| Diplocynodon sp. | 0.23 | semi-aquatic | n.a. | yes | Eocene–Miocene | IPB R144 ⁄ 1 |
| Diplocynodon remensis | 0.24 | semi-aquatic | nuchal | yes | Thanetian | MNHN. F. No number |
| Machimosaurus hugii | 0.22 | semi-aquatic | n.a. | yes | Late Jurassic | SMNS 81608 |
| Sarcosuchus imperator | 0.24 | semi-aquatic | n.a. | yes | Upper Cretaceous | MNHN.F. GDF 380 |
| Steneosaurus sp. | 0.07 | semi-aquatic | n.a. | yes | Late Jurassic | NMS 752 |
| Steneosaurus jugleri | 0.12 | semi-aquatic | n.a. | yes | Late Jurassic | NMS 7152 |
| Paleosuchus trigonatus | 0.29 | semi-aquatic | n.a. | yes | extant | MCL 420003939 |
| Protocaiman peligrensis | 0.13 | semi-aquatic | n.a. | yes | Danian | UCMP 131693 |
| Teleosaurus cadomensis | 0.22 | semi-aquatic | n.a. | yes | Bathonian | MNHN Histo 1960 |
| Goniopholis sp. | 0.2 | semi-aquatic | n.a. | yes | Oxfordian-Berriasian | MNHN Histo 1727 |
| Borealosuchus sp. | 0.17 | semi-aquatic | n.a | yes | Campanian-Ypresian | UCMP 133903 |
| Mahajangasuchus insignis | 0.17 | semi-aquatic | n.a. | yes | Campanian | UA 9962 |
| Brachychampsa montana | 0.22 | semi-aquatic | n.a. | yes | Maastrichtian | UCMP 133901 |
| Osteolaemus tetraspis | 0.12 | semi-aquatic | n.a. | yes | extant | MNHN-AC-1991.4488 |
| Paleosuchus palpebrosus | 0.11 | semi-aquatic | n.a. | yes | extant | MNHN.AC-1909.204 |
| Caiman crocodilus | 0.27 | semi-aquatic | nuchal | yes | extant | Sorbonne Université - NA |
| Borealosuchus wilsoni | 0.18 | semi-aquatic | n.a. | yes | Ypresian | UCMP 131696 |
| Paratypothorax sp. | 0.25 | terrestrial | paramedian | yes | Late Triassic | PEFO 5030 |
| Aetosaurus scagliai | 0.05 | terrestrial | paramedian | yes | Late Triassic | PVL 2073 |
We sampled 32 cross sections of pseudosuchian osteoderms. The taxa were categorized based on two different lifestyles: terrestrial and semi-aquatic (table 1b). We decided not to distinguish the marine animals from the freshwater semi-aquatic forms as they have a similar amphibious ambush predator lifestyle [43,44]. The pelagic marine forms from the Jurassic (the metryorhinchids) [45,46] had completely lost the osteoderm shield and are therefore not suitable for this study (this aspect is discussed below). Extinct pseudosuchians are categorized based on the orientation of their skull neurosensory organs and limb postures as reviewed in a previous article [47].
(b). Data acquisition
We produced photographs of each cross section before segmenting and rendering them binary in black and white with Adobe Photoshop CC 2015 (electronic supplementary material, S2 and S3), so that the bone matrix appears in black and the empty cavities appear in white. The ornamentation of dermal bones is often made of crests separated by pits, which systematically host large clusters of blood vessels from the soft dermis. These blood vessels are intimately associated with the dermal bone vascularization as they pierce its surface [39] and connect vessel clusters in the internal cavities (spongiosa) via these vascular openings at the surface of the bone [37,39]. As pseudosuchians [48], tryonichids [37] and helochelydrids [41] possess ornamented post-cranial dermal bones whose dermis vascularization is directly related to the dermal bone internal vascularization. Although these pits are large, they obviously do not over-estimate the porosity of the bone as they are fully filled in by a great number of blood vessels [39]. For that reason, we decided to include the space of these pits into our measurements. To do so, we connected the top of the crests of the ornamentation with a virtual black line of one pixel in the most parsimonious way to embed the surface of the pits into the vascular measurements (electronic supplementary material, S3). We exported the pictures in TIFF format (electronic supplementary material, S2) and analysed them with Bone profiler [49] in order to measure the area occupied by the empty spaces proportionally to the entire area covered by bone and vascular spaces (as detailed in electronic supplementary material, S3).
(c). Phylogenetic comparative analyses
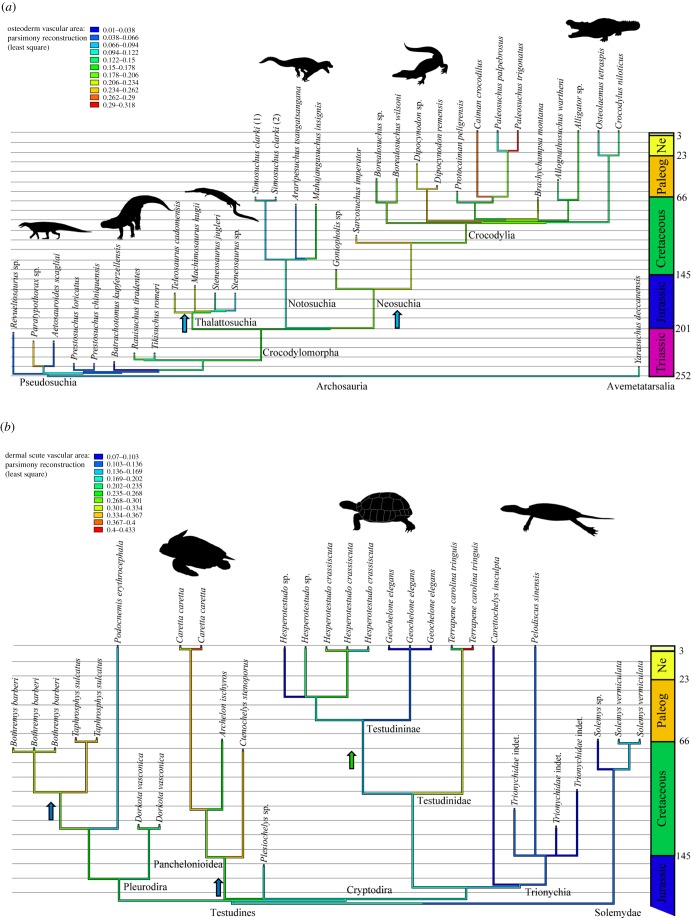
For both Pseudosuchia and Testudinata, time-scaled phylogenetic relationships of the sampled specimens were reconstructed in Mesquite [50] by relying on published references [5,41,42,51–63] (figure 1). We then traced the evolution of the post-cranial dermal bone vascular area using the least-squared parsimony to calculate the ancestral states for each clade. In order to test the influence of the phylogeny (i.e. the historical constraint) on the vascular area of the post-cranial dermal skeleton, we exported the trees in NEX format into R [64] and we further computed both Pagel's λ [65] and Blomberg's K [66] after uploading the ‘caper’ package [67,68]. Finally, we tested the correlation between the post-cranial dermal bone vascular area and the corresponding lifestyle for each taxon using a phylogenetic ANOVA. This is a statistical test that reveals a correlation between quantitative and qualitative data while retracting the influence of the phylogeny, which is quantified either by K or λ—we decided to consider both options [68] (table 2).
Figure 1.
(a) Reconstruction of the osteoderm vascular area on the phylogeny of Pseudosuchia using a least-square reconstruction. The phylogeny was reconstructed and time-scaled according to published references [5,51–58]. The light blue arrows represent the transitions from a terrestrial to a semi-aquatic lifestyle. (b) Reconstruction of the dermal scute vascular area on the phylogeny of Testudinata using a least-square reconstruction. The phylogeny was reconstructed and time-scaled according to published references [41,42,59–63]. The dark blue arrows represent the transitions from a freshwater to a marine lifestyle. The green arrow represents a transition from a freshwater to a terrestrial lifestyle. Regarding the dermal plates, which belong to the same species or specimen, we decided to separate them from a 1 Myr-old last hypothetical common ancestor: a systematic error which is below 1% when considering the total branch length within the phylogeny timescale (180 Ma for the testudinatans and 250 Ma for the pseudosuchians). Paleog, Paleogene, Ne, Neogene.
Table 2.
Statistical results. s.d.: standard deviation; max: maximum value; min: minimum value.
| Pseudosuchia |
Testudinata |
||||
|---|---|---|---|---|---|
| phylogenetic analyses | result/value | p-value | phylogenetic analyses | result/value | p-value |
| K | significant/K = 0.41 | 0.004 | K | significant/K = 0.09 | 0.003 |
| ANOVA(K) | non-significant | 0.21 | ANOVA(K) | non-significant | 0.6658 |
| λ | significant/λ = 0.99 | 1.97 × 10−5 | λ | significant/λ = 0.83 | 2.48 × 10−5 |
| ANOVA(λ) | non-significant | 0.21 | ANOVA(λ) | non-significant | 0.6658 |
| statistical results |
statistical results |
||||
|---|---|---|---|---|---|
| lifestyle | terrestrial | semi-aquatic | terrestrial | freshwater | marine |
| mean porosity | 0.09 | 0.18 | 0.18 | 0.14 | 0.31 |
| median | 0.06 | 0.19 | 0.16 | 0.11 | 0.33 |
| s.d. | 0.07 | 0.06 | 0.10 | 0.06 | 0.06 |
| min | 0.01 | 0.07 | 0.07 | 0.09 | 0.18 |
| max | 0.25 | 0.29 | 0.40 | 0.24 | 0.39 |
3. Results
(a). Evolution of vascular density in the osteoderms of Pseudosuchia
We first tested whether the variability of the vascular area—proportionally to the dermal bone area—was inherited from the phylogenetic relationships of the studied species. Phylogenetic tests showed that the vascular area in the osteoderms of the pseudosuchians is significantly influenced by the phylogeny, as both the Blomberg's K and the Pagel's λ tests are significant (p-values of less than 0.05; table 2). The fact that the λ-value of 0.99 is very close to the maximum (λ = 1) means that the vascular area covaries in direct proportion with the species' shared evolutionary history through a Brownian motion on the phylogeny [65,69]. The K-test shows a significant p-value (table 2) and thus emphasizes the tendency of closely related species to share a similar osteoderm vascular area. Nevertheless, as the K-value itself clearly remains below 1, the phylogeny must not be the only component that explains the resulting evolutionary pattern of vascular area variability within Pseudosuchia [66,70].
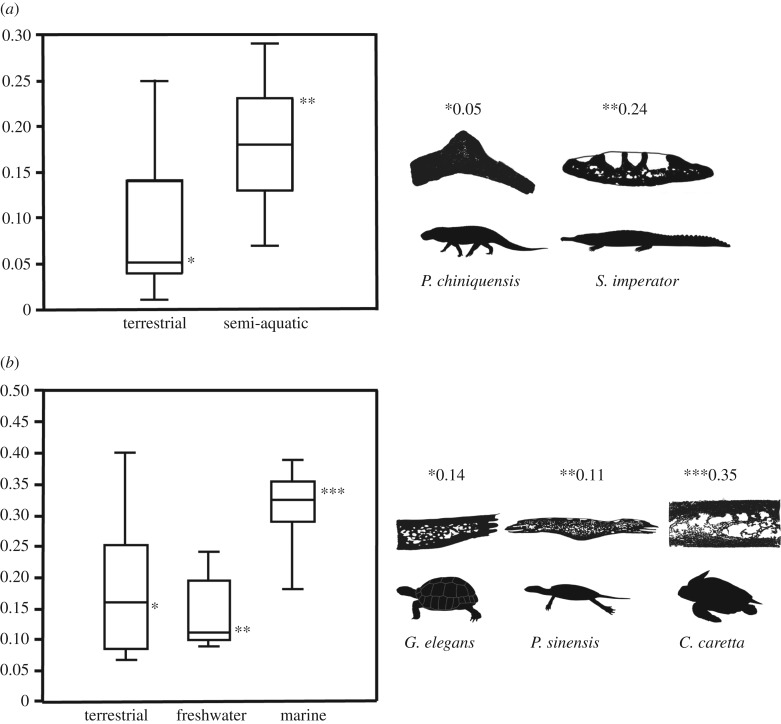
A first glimpse of the vascular density distribution would suggest that the lifestyle of the studied taxa could partly explain this variability. Boxplots were calculated to illustrate the distribution of the vascular cross-sectional area in the osteoderms of semi-aquatic and terrestrial pseudosuchians. Semi-aquatic forms exhibit a larger vascular area (proportionally to their dermal bone area) than terrestrial pseudosuchians (while showing a more pronounced apical ornamentation; table 2 and figure 2a; electronic supplementary material, S2). Indeed, although both datasets show an equal standard deviation (s.d.terrestrial = 0.07; s.d.semi-aquatic = 0.06), the mean value of the osteoderm vascular area is equal to 0.09 in the terrestrial forms, whereas it is twice as high in the semi-aquatic pseudosuchians (mean = 0.18).
Figure 2.
(a) Boxplot of the osteoderm vascular area in the pseudosuchians. (b) Boxplot of the dermal scute vascular area in the testudinatans. The four quartiles represent the dispersion of the values for each lifestyle.
In order to test whether this distribution could partly explain the variability of vascular density in Pseudosuchia, we performed a phylogenetic ANOVA that takes into account the phylogenetic signal. The results present no significant correlation between the osteoderm vascular area and the pseudosuchian lifestyle (table 2).
(b). Evolution of vascular density in the shell of Testudinata
We evaluated the vascular areas of turtle shell in a phylogenetic context. They show that the dermal shell bone vascular density is significantly influenced by the phylogeny since both the Blomberg's K and the Pagel's λ tests are significant (p-values of less than 0.05; table 2). However, both the λ and the K show lower values (λ = 0.83; K = 0.09) than for the pseudosuchian osteoderm vascular area (λ = 0.99; K = 0.41). We deduce that the phylogeny explains to a lesser extent the variability of shell vascular density in testudinatans than in pseudosuchians (figure 1b).
As presented with boxplots (figure 2b) and in table 2, the testudinatan dermal bone vascular area seems to score higher values than in the pseudosuchian osteoderms although the standard deviation is equal, with the exception of terrestrial testudinatans, whose vascular density varies in a larger spectrum around a mean value of 0.18 (s.d. = 0.10). Unlike semi-aquatic pseudosuchians (which are most often found in freshwater environments), the freshwater turtle dermal bones show a lower vascular density (mean = 0.14) than terrestrial forms (mean = 0.18). The presence of ornamentation in both Trionychia and in Helochelydridae does not seem to influence the global turtle dermal bone vascularity as these bones still score a low vascular area (all values are lower than 0.16; table 2; see Material and methods). As a third category, the marine turtles, which are fully aquatic (with a very brief terrestrial excursion on land for females to lay eggs), present a high average value of shell vascular area (mean = 0.31) with a standard deviation similar to that of the freshwater turtles (figure 2b and table 2).
A phylogenetic ANOVA was performed and shows that the vascular area in the testudinatan post-cranial dermal bones was not significantly correlated with their lifestyle (table 2), despite these discrete boxplot distributions.
4. Discussion
(a). Pseudosuchian osteoderm vascularization: historical constraints versus ecophysiological adaptations
Our results show that the variability of the osteoderm vascularization correlates with the phylogenetic relationships within Pseudosuchia. Although the lifestyle seems to partly explain the rest of the correlation factor according to the global distribution of the data, our phylogenetic ANOVA revealed no significant correlation between osteoderm vascular variability and lifestyle. The high osteoderm vascularity in the semi-aquatic forms was therefore likely the result of a historical constraint (as evidenced by the significant values of λ and K) rather than an ecological adaptation based on natural selection. Nevertheless, some recent studies on living species have shown that the bone cavities in the crocodylian osteoderms reveal an enclosed vascular proliferation [39], which is involved in acidosis buffering during prolonged apnea [30], as well as in heat transfer during emerged and semi-emerged basking periods [23]. Therefore, we cannot refute the existence of such physiological mechanisms in the extinct crocodylomorphs who shared the same semi-aquatic ambush predator behaviour as the extant crocodylians: the extinct neosuchians (e.g. Sarcosuchus imperator, Goniopholis sp.) [31,71,72] and the teleosaurids [45,55,73].
Regarding the thalattosuchians that adopted a pelagic lifestyle involving long-term apneas (Metriorhynchidae; [44–46]), the loss of the dermal shield must have negatively impacted their performance in bone acidosis buffering. Nevertheless, other pathways can buffer acidosis via the involvement of soft tissues [74]. Such mechanisms have already been observed in marine birds and mammals [75–77]. Contrary to extant crocodylians, marine birds and mammals are very active swimmers. Their lack of oxygen due to apnea essentially affects their appendicular musculature. To compensate for the acidity increase, limb muscles synthetize a protein (carnosine) [78–80] which complexes with protons and thus buffers the intracellular acidosis in muscle tissues where free oxygen concentration is the lowest. As the fossil forms—metriorhynchids—probably were active sea predators, as evidenced by the presence of a tail fluke and swimming paddles [45,46], we can assume that metabolic acidosis buffering could have involved the muscular system as in extant marine birds and mammals. However, the reasons for the metriorhynchids to have lost their osteoderm shield remain unknown. This loss could reflect a complex conjuncture involving both phylogenetic and structural constraints influencing the development of the dorsal shield in disregard of its physiological implication(s) (i.e. weight loss, flexibility along the anteroposterior axis, etc. [45,46,81]).
(b). Testudinatan shell vascularization: historical constraints versus ecophysiological adaptations
Likewise, our results show that the testudinatan shell vascular density is essentially constrained by the phylogeny despite the noticeable differences in the mean values of vascular area between taxa belonging to different lifestyle categories (table 2 and figure 2b).
Higher porosity is encountered in the marine forms. It probably provides a dense vascular system, which facilitates long-term apnea via bone acidosis buffering since this function is essential to sea turtles, of which only the females emerge on land for nesting [82]. Most of their feeding habits rely on a vegetarian or omnivorous diet from the sea bottom [83]. Density reduction due to the lightening of the shell bone perforated by a large number of vascular canals obviously increases their buoyancy and intensifies their effort to dive and remain at the bottom of the sea. Because the control of buoyancy is moderated by the lungs [84,85], we strongly suspect that the porosity of the shell could be better explained as the result of physiological functions such as bone acidosis buffering than in relation to biomechanics.
Unlike the marine forms, the freshwater turtles do not exhibit a high shell bone porosity although they are known to perform bone acidosis buffering during prolonged apnea and while hibernating in freezing and/or anoxic conditions [24–30,86]. Some freshwater species such as the trionychids seem to have developed a different strategy to withstand long duration apneas. Indeed, in comparison with the other freshwater testudinatans, the trionychids are known to have a lower performance in bone acidosis buffering as they are less tolerant to anoxia [87]. Instead, they exchange blood gases with those dissolved in the surrounding water using pharyngeal, cloacal and cutaneous respiration [88]. Although gas exchanges are not possible through scales of keratin in sauropsids (including crocodylians and testudinatans), this mechanism is rendered possible in trionychids by the secondary loss of their superficial keratin layer [63]. It is worth mentioning that the use of cutaneous respiration in Trionychia correlates with a rare expression of shell apical ornamentation in testudines. As illustrated in previous studies [37], the pits which compose the trionychid shell sculpture always house one or several vascular openings which provide a proliferation of superficial vessels as in crocodylian ornamented osteoderms [39]. This configuration provides a large blood-vessel network for gas exchanges in cutaneous respiration [89].
Even if heat exchange with the environment is vital for testudinatans [90], which are ectotherms, this function does not seem to correlate with the evolutionary pattern of shield vascularization (considering the vascular area as a proxy). Indeed, both freshwater and terrestrial turtle dermal bones globally show a lower vascular density than marine forms, although temperature variations in the sea are much narrower than on land or in freshwater environments.
In conclusion, the dermal shield of the testudinatans seems to play multiple physiological roles which differently concern: (1) marine turtles (acidosis buffering during prolonged apnea); (2) freshwater turtles (cutaneous respiration and/or acidosis buffering in response to prolonged apnea or hibernation in anoxic or freezing conditions, heat transfer when basking); and (3) terrestrial tortoises (heat transfer). Therefore, it seems unlikely that any resulting combination of these functions represents the primary determinant of morphology once we have considered the influence of the phylogenetic relationships (historical constraints).
(c). Evolutionary trends in Pseudosuchia and Testudinata
Pseudosuchians and testudinatans have been repeatedly defined as sister taxa according to several phylogenetic reconstructions [91–97]. Among amniotes, these two groups are the main ones to have developed a large post-cranial dermal skeleton which is known to be used in both acidosis buffering and heat transfer. This pattern leads us to wonder if this ability of the post-cranial dermal bones to perform ecophysiological functions results from a phylogenetic heritage or consists of a functional analogy. The vascular density in the post-cranial dermal skeleton increased when pseudosuchians transited to a semi-aquatic lifestyle in the Early Jurassic and when turtles transited to a marine lifestyle (within Pleurodira during the Cretaceous, within Cryptodira during the Jurassic and maybe in some early testudinatan species such as Eileanchelys waldmani and Heckerochelys romani for which the assumed marine lifestyle is still debated [38,98]; figures 1 and 2). All these transitions probably induced bone acidosis buffering to balance prolonged apnea, which directly depends on the bone vascularization inside the shell cavities [29].
Even though the vascularization in post-cranial dermal bones must also be involved in heat transfer due to its peripheral location on the body [20,35], testudinatans and pseudosuchians nevertheless have evolved through very different thermal metabolism patterns. Indeed, the pseudosuchians derive from an endothermic ancestor [99–103] whereas ectothermy is a plesiomorphic condition in Testudinata [38,104]. The increase in dermal bone vascularization relates to heat transfer in the semi-aquatic crocodylomorphs [23,39,47] but this process does not explain the observed pattern in the testudinatan shell as the terrestrial forms score lower relative vascular area although they are the most exposed to external thermal variations.
The functional role(s) of bone ornamentation may also differ between the turtles and the crocodylians. Indeed, even though the vascular openings within the ornamental pits must play a role in cutaneous respiration in soft-shell turtles, this function is not possible in the crocodylians since their entire body is covered by a layer of keratine [31]. The function(s) of bone ornamentation in Crocodylomorpha must instead concern acidosis buffering and heat transfer via the housing of vessel clusters straight over the bone apical surface in connection with the blood vessels underneath, which are enclosed in the bone cavities within the osteoderm core (spongiosa).
As a conclusion, we suggest that the vascular plasticity of the post-cranial dermal bones in both Testudinata and Pseudosuchia probably helped these clades make major evolutionary shifts by offering various pathways to oxygen and/or heat management. Despite the fact that upshifts in vascular density often relate to an increased frequency of internal low oxygen due to a freshwater or marine lifestyle, we do not exclude that vascular density also relates to other vital functions as well as historical and structural constraints which drive the development and morphology of the dermal plates [105]. The complexity of multi-functional roles of the post-cranial dermal skeleton in both pseudosuchians and testudinatans might be a reason why our phylogenetic ANOVA revealed no relation between ‘vascular area’ and ‘ecology’ despite obvious differences between the lifestyle categories. Our results however demonstrate that the advanced development of a post-cranial skeleton in these groups was crucial for the survival and dispersal of these taxa in various ecological niches. This major evolutionary step should be more thoroughly investigated.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Damien Germain (curator of the paleohistology collection in the Muséum National d’ Histoire Naturelle; MNHN) for giving us access to the sample of European pseudosuchian specimens as well as the curators of the museums and collections who granted access to materials used in the previous studies on the histology of armoured animals.
Data accessibility
The data are accessible in electronic supplementary material files.
Authors' contributions
F.C. computed and analysed the data before writing the first version of the manuscript. S.S. funded this study and gave important input regarding the interpretation of the data. T.M.S. provided his expertise on the testudinatan anatomy and phylogenetic relationships. J.B.D. and I.A.C. provided their expertise on the histology and on the natural history of the pseudosuchians. All authors worked on the finalization of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a grant from the Vetenskapsrådet awarded to S.S. (grant no. 2015–04335). T.M.S. acknowledges support by the Swiss National Science Foundation (grant no. 205321_162775).
References
- 1.Dubansky BH, Dubansky BD. 2018. Natural development of dermal ectopic bone in the American alligator (Alligator mississippiensis) resembles heterotopic ossification disorders in humans. Anat. Rec. 301, 56–76. ( 10.1002/ar.23682) [DOI] [PubMed] [Google Scholar]
- 2.Gilbert SF, Loredo GA, Brukman A, Burke AC. 2001. Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol. Dev. 3, 47–58. ( 10.1046/j.1525-142x.2001.003002047.x) [DOI] [PubMed] [Google Scholar]
- 3.Vickaryous MK, Hall BK. 2008. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J. Morphol. 269, 398–422. ( 10.1002/jmor.10575) [DOI] [PubMed] [Google Scholar]
- 4.Vickaryous MK, Sire JY. 2009. The integumentary skeleton of tetrapods: origin, evolution and development. J. Anat. 214, 441–464. ( 10.1111/j.1469-7580.2008.01043.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesbitt SJ. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 352, 1–292. ( 10.1206/352.1) [DOI] [Google Scholar]
- 6.Cerda IA, Desojo JB, Trotteyn MJ, Scheyer TM. 2015. Osteoderm histology of Proterochampsia and Doswelliidae (Reptilia: Archosauriformes) and their evolutionary and paleobiological implications. J. Morphol. 276, 385–402. ( 10.1002/jmor.20348) [DOI] [PubMed] [Google Scholar]
- 7.Cerda IA, Desojo JB. 2011. Dermal armour histology of aetosaurs (Archosauria: Pseudosuchia), from the Upper Triassic of Argentina and Brazil. Lethaia 44, 417–428. ( 10.1111/j.1502-3931.2010.00252.x) [DOI] [Google Scholar]
- 8.Irmis RB, Nesbitt SJ, Sues HD. 2013. Early Crocodylomorpha. Geol. Soc. London Spec. Publ. 379, 275–302. ( 10.1144/SP379.24) [DOI] [Google Scholar]
- 9.Burns ME, Vickaryous MK, Currie PJ. 2013. Histological variability in fossil and recent alligatoroid osteoderms: systematic and functional implications. J. Morphol. 274, 676–686. ( 10.1002/jmor.20125) [DOI] [PubMed] [Google Scholar]
- 10.Anjan B, Bhullar S. 2008. Osteoderms of the California legless lizard Anniella (Squamata: Anguidae) and their relevance for considerations of miniaturization. Copeia 4, 785–793. ( 10.1643/CG-07-189) [DOI] [Google Scholar]
- 11.Acrai B, Wagner HD. 2013. Micro-structure and mechanical properties of the turtle carapace as a biological composite shield. Acta Biomater. 9, 5890–5902. ( 10.1016/j.actbio.2012.12.023) [DOI] [PubMed] [Google Scholar]
- 12.Chen IH, Yang W, Meyers MA. 2015. Leatherback sea turtle shell: a tough and flexible biological design. Acta Biomater. 28, 2–12. ( 10.1016/j.actbio.2015.09.023) [DOI] [PubMed] [Google Scholar]
- 13.Vickaryous MK, Hall BK. 2006. Osteoderm morphology and development in the nine-banded armadillo, Dasypus novemcinctus (Mammalia, Xenarthra, Cingulata). J. Morphol. 267, 1273–1283. ( 10.1002/jmor.10475) [DOI] [PubMed] [Google Scholar]
- 14.Wolf D, Kalthoff DC, Sander PM. 2012. Osteoderm histology of the Pampatheriidae (Cingulata, Xenarthra, Mammalia): implications for systematics, osteoderm growth, and biomechanical adaptation. J. Morphol. 273, 388–404. ( 10.1002/jmor.11029) [DOI] [PubMed] [Google Scholar]
- 15.Scheyer TM, Sander PM. 2004. Histology of ankylosaur osteoderms: implications for systematics and function. J. Vertebr. Paleontol. 24, 874–893. ( 10.1671/0272-4634) [DOI] [Google Scholar]
- 16.Main RP, de Ricqlès A, Horner JR, Padian K. 2005. The evolution and function of thyreophoran dinosaur scutes: implications for plate function in stegosaurs. Paleobiology 31, 291–314. ( 10.1666/0094-8373) [DOI] [Google Scholar]
- 17.Farlow JO, Hayashi S, Tattersall GJ. 2010. Internal vascularity of the dermal plates of Stegosaurus (Ornithischia, Thyreophora). Swiss. J. Geosci. 103, 173–185. ( 10.1007/s00015-010-0021-5) [DOI] [Google Scholar]
- 18.Carrano MT, D'Emic MD. 2015. Osteoderms of the titanosaur sauropod dinosaur Alamosaurus sanjuanensis Gilmore, 1922. J. Vertebr. Paleontol. 35, e901334 ( 10.1080/02724634.2014.901334) [DOI] [Google Scholar]
- 19.Sun CY, Chen PY. 2013. Structural design and mechanical behavior of alligator (Alligator mississippiensis) osteoderms. Acta Biomater. 9, 9049–9064. ( 10.1016/j.actbio.2013.07.016) [DOI] [PubMed] [Google Scholar]
- 20.Chen IH, Yang W, Meyers MA. 2014. Alligator osteoderms: mechanical behavior and hierarchical structure. Mater. Sci. Eng. C 35, 441–448. ( 10.1016/j.msec.2013.11.024) [DOI] [PubMed] [Google Scholar]
- 21.Broeckhoven C, Diedericks G, Mouton PLFN. 2015. What doesn't kill you might make you stronger: functional basis for variation in body armour. J. Anim. Ecol. 84, 1213–1221. ( 10.5061/dryad.7h4r3) [DOI] [PubMed] [Google Scholar]
- 22.Du Plessis A, Broeckhoven C, Yadroitsev I, Yadroitsava I, le Roux SG. 2018. Analyzing nature's protective design: the glyptodont body armor. J. Mech. Behav. Biomed. 82, 218–223. ( 10.1016/j.jmbbm.2018.03.037) [DOI] [PubMed] [Google Scholar]
- 23.Clarac F, Quilhac A. 2019. The crocodylian skull and osteoderms: a functional exaptation to ectothermy? Zoology 132, 31–40. ( 10.1016/j.zool.2018.12.001) [DOI] [PubMed] [Google Scholar]
- 24.Jackson DC, Heisler N. 1982. Plasma ion balance in submerged anoxic turtles at 3°C: the role of calcium lactate formation. Res. Physiol. 49, 159–174. ( 10.1016/0034-5687(82)90071-8) [DOI] [PubMed] [Google Scholar]
- 25.Jackson DC, Goldberger Z, Visuri S, Armstrong RN. 1999. Ionic exchanges of turtle shell in vitro and their relevance to shell function in the anoxic turtle. J. Exp. Biol. 202, 503–520. [DOI] [PubMed] [Google Scholar]
- 26.Jackson DC. 2000. Living without oxygen: lessons from the freshwater turtle. Comp. Biochem. Physiol. A 125, 299–315. ( 10.1016/S1095-6433(00)00160-4) [DOI] [PubMed] [Google Scholar]
- 27.Jackson DC, Crocker CE, Ultsch GR. 2000. Bone and shell contribution to lactic acid buffering of submerged turtles Chrysemys picta bellii at 3°C. Am. J. Physiol. Reg. Integr. Comp. Physiol. 278, R1564–R1571. ( 10.1152/ajpregu.2000.278.6.r1564) [DOI] [PubMed] [Google Scholar]
- 28.Jackson DC, Ramsey AL, Paulson JM, Crocker CE, Ultsch GR. 2000. Lactic acid buffering by bone and shell in anoxic softshell and painted turtles. Phys. Chem. Zool. 73, 290–297. ( 10.1086/316754) [DOI] [PubMed] [Google Scholar]
- 29.Jackson DC. 2004. Surviving extreme lactic acidosis: the role of calcium lactate formation in the anoxic turtle. Resp. Physiol. Neurobiol. 144, 173–178. ( 10.1016/j.resp.2004.06.020) [DOI] [PubMed] [Google Scholar]
- 30.Jackson DC, Andrade D, Abe AS. 2003. Lactate sequestration by osteoderms of the broad-nose caiman, Caiman latirostris, following capture and forced submergence. J. Exp. Biol. 206, 3601–3606. ( 10.1242/jeb.00611) [DOI] [PubMed] [Google Scholar]
- 31.Trutnau L, Sommerlad R. 2006. Crocodilians their natural history and captive husbandry. Frankfurt am Main, Germany: Edition Chimaira. [Google Scholar]
- 32.Seidel MR. 1979. The osteoderms of the American alligator and their functional significance. Herpetol. Leag. 35, 375–380. [Google Scholar]
- 33.Seebacher F, Franklin CE. 2004. Integration of autonomic and local mechanisms in regulating cardiovascular responses to heating and cooling in a reptile (Crocodylus porosus). J. Comp. Physiol. B 174, 577–585. ( 10.1007/s00360-004-0446-0) [DOI] [PubMed] [Google Scholar]
- 34.Seebacher F, Franklin CE. 2007. Redistribution of blood within the body is important for thermoregulation in an ectothermic vertebrate (Crocodylus porosus). J. Comp. Physiol. B 177, 841–848. ( 10.1007/s00360-007-0181-4) [DOI] [PubMed] [Google Scholar]
- 35.Grigg GC, Alchin J. 1976. The role of the cardiovascular system in thermoregulation of Crocodylus johnstoni. Physiol. Zool. 49, 24–36. ( 10.1086/physzool.49.1.30155674) [DOI] [Google Scholar]
- 36.Tattersall DJ, Cadena V. 2010. Insights into animal temperature adaptations revealed through thermal imaging. Imaging Sci. J. 58, 261–268. ( 10.1179/136821910X12695060594165) [DOI] [Google Scholar]
- 37.Scheyer TM, Sander PM, Joyce WG, Böhme W, Witzel U. 2007. A plywood structure in the shell of fossil and living soft-shelled turtles (Trionychidae) and its evolutionary implications. Org. Divers. Evol. 7, 136–144. ( 10.1016/j.ode.2006.03.002) [DOI] [Google Scholar]
- 38.Scheyer TM, Sander PM. 2007. Carapace bone histology in the giant pleurodiran turtle Stupendemys geographicus: phylogeny and function. Proc. R. Soc. B 274, 1885–1893. ( 10.1098/rspb.2007.0499) [DOI] [Google Scholar]
- 39.Clarac F, Buffrénil V, Cubo J, Quilhac A. 2018. Vascularisation in ornamented osteoderms: physiological implications in ectothermy and amphibious lifestyle in the crocodylomorphs? Anat. Rec. 301, 175–183. ( 10.1002/ar.23695) [DOI] [PubMed] [Google Scholar]
- 40.Buffrénil V, Clarac F, Fau M, Martin S, Martin B, Pellé E, Laurin M. 2015. Differentiation and growth of bone ornamentation in vertebrates: a comparative histological study among the Crocodylomorpha. J. Morphol. 276, 425–445. ( 10.1002/jmor.20351) [DOI] [PubMed] [Google Scholar]
- 41.Joyce WG, Chapman SD, Moody RTJ, Walker CA. 2011. The skull of the solemydid turtle Helochelydra nopcsai from the Early Cretaceous of the Isle of Wight (UK) and a review of Solemydidae. Palaeontology 86, 75–97. ( 10.1111/j.1475-4983.2011.01075.x) [DOI] [Google Scholar]
- 42.Joyce WG. 2017. A review of the fossil record of basal Mesozoic turtles. Bull. Peabody Mus. Nat. Hist. 58, 65–113. ( 10.3374/014.058.0105) [DOI] [Google Scholar]
- 43.Young MT, de Andrade MB, Cornéed JJ, Steel L, Foffa D. 2014. Re-description of a putative Early Cretaceous ‘teleosaurid’ from France, with implications for the survival of metriorhynchids and teleosaurids across the Jurassic-Cretaceous Boundary. Hist. Biol. 27, 947–953. ( 10.1016/j.annpal.2014.01.002) [DOI] [Google Scholar]
- 44.Fanti F, Miyashita T, Cantelli L, Mnasri F, Dridi J, Contessi M, Cau A. 2016. The largest thalattosuchian (Crocodylomorpha) supports teleosaurid survival across the Jurassic-Cretaceous boundary. Cret. Res. 61, 263–274. ( 10.1016/j.cretres.2015.11.011) [DOI] [Google Scholar]
- 45.Buffetaut E. 1982. Radiation évolutive, paléoécologie et biogéographie des crocodiliens mésosuchiens. Mém. Soc. Géol. France N S 142, 1–88. [Google Scholar]
- 46.Young MT, Brusatte SL, Ruta M, de Andrade MB. 2010. The evolution of Metriorhynchoidea (Mesoeucrocodylia, Thalattosuchia): an integrated approach using geometric morphometrics, analysis of disparity, and biomechanics. Zool. J. Linnean Soc. 158, 801–859. ( 10.1111/j.1096-3642.2009.00571.x) [DOI] [Google Scholar]
- 47.Clarac F, de Buffrénil V, Brochu CA, Cubo J. 2017. The evolution of bone ornamentation in Pseudosuchia: morphological constraints versus ecological adaptation. Biol. J. Linn. Soc. 121, 395–408. ( 10.1093/biolinnean/blw034) [DOI] [Google Scholar]
- 48.Clarac F, Souter T, Cornette R, Cubo J, de Buffrénil V. 2015. A quantitative assessment of bone area increase due to ornamentation in the Crocodylia. J. Morphol. 276, 1183–1192. ( 10.1002/jmor.20408) [DOI] [PubMed] [Google Scholar]
- 49.Girondot M, Laurin M. 2003. Bone Profiler: a tool to quantify, model, and statistically compare bone-section compactness profiles. J. Vertebr. Paleontol. 23, 458–461. ( 10.1671/0272-4634(2003)023[0458:bpattq]2.0.co;2) [DOI] [Google Scholar]
- 50.Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis. Version 2.75. See http://mesquiteproject.org.
- 51.Jouve S. 2009. The skull of Teleosaurus cadomensis (Crocodylomorpha; Thalattosuchia). J. Vertebr. Paleontol. 29, 88–102. ( 10.1671/039.029.0129) [DOI] [Google Scholar]
- 52.de Andrade MB, Edmonds R, Benton MJ, Schouten R. 2011. A new Berriasian species of Goniopholis (Mesoeucrocodylia, Neosuchia) from England, and a review of the genus. Zool. J. Linnean Soc. 163, S66–S108. ( 10.1111/j.1096-3642.2011.00709.x) [DOI] [Google Scholar]
- 53.Pol D, Nascimento PM, Carvahlo AB, Riccomini C, Pires-Domingue RA, Zaher H. 2014. A new notosuchian from the late Cretaceous of Brazil and the phylogeny of advanced notosuchians. PLoS ONE 9, e93105 ( 10.1371/journal.pone.0093105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pol D, Leardi JM. 2015. Diversity patterns of Notosuchia (Crocodyliformes, Mesoeucrocodylia) during the Cretaceous of Gondwana. In Reptiles extintos-volumen en homenaje a zulma a gasparini. Publicacion electronica de la asociacion paleontologica Argentina 15(1) (eds Fernandez M, Herrera Y), pp. 172–186. Buenos Aires, Argentina: Asociación Paleontológica Argentina. [Google Scholar]
- 55.Young MT, et al. 2014. Revision of the Late Jurassic teleosaurid genus Machimosaurus (Crocodylomorpha, Thalattosuchia). R. Soc. open sci. 1, 140222 ( 10.1098/rsos.140222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilberg EW. 2015. What's in an outgroup? The impact of outgroup choice on the phylogenetic position of Thalattosuchia (Crocodylomorpha) and the origin of Crocodyliformes. Syst. Biol. 64, 621–637. ( 10.1093/sysbio/syv020) [DOI] [PubMed] [Google Scholar]
- 57.Nesbitt SJ, et al. 2019. The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature 544, 484–544. ( 10.1038/nature22037) [DOI] [PubMed] [Google Scholar]
- 58.Bona P, Ezcurra MD, Barrios F, Blanco MVF. 2018. A new Paleocene crocodylian from southern Argentina sheds light on the early history of caimanines. Proc. R. Soc. B 285, 20180843 ( 10.1098/rspb.2018.0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meylan PA, Sterrer W. 2000. Hesperotestudo (Testudines: Testudinidae) from the Pleistocene of Bermuda, with comments on the phylogenetic position of the genus. Zool. J. Linnean. Soc. 128, 51–76. ( 10.1006/zjls.1998.0199) [DOI] [Google Scholar]
- 60.Kear BP, Lee MSY. 2006. A primitive protostegid from Australia and early sea turtle evolution. Biol. Lett. 2, 116–119. ( 10.1098/rsbl.2005.0406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joyce WG, Parham JF, Lyson TR, Warnock RCM, Donoghue PCJ. 2013. A divergence dating analysis of turtles using fossil calibrations: an example of best practices. J. Paleontol. 87, 612–634. ( 10.1666/12-149) [DOI] [Google Scholar]
- 62.Sterli J, Pol D, Laurin M. 2013. Incorporating phylogenetic uncertainty on phylogeny-based palaeontological dating and the timing of turtle diversification. Cladistics 9, 232–246. ( 10.1111/j.1096-0031.2012.00425.x) [DOI] [PubMed] [Google Scholar]
- 63.Nakajima Y, Danilov IG, Hirayama R, Sonoda T, Scheyer TM. 2017. Morphological and histological evidence for the oldest known softshell turtles from Japan. J. Vertebr. Paleontol. 37, e1278606 ( 10.1080/02724634.2017.1278606) [DOI] [Google Scholar]
- 64.R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for statistical Computing; (http://www.r-project.org/) [Google Scholar]
- 65.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 66.Blomberg SP, Garland T. 2002. Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J. Evol. Biol. 15, 899–910. ( 10.1046/j.1420-9101.2002.00472.x) [DOI] [Google Scholar]
- 67.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2012. The caper package: comparative analysis of phylogenetics and evolution in R. R package version 0.5.2. London, UK: CRAN.R Project. [Google Scholar]
- 68.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 69.Garland T Jr, Dickerman AW, Janis CM, Jason AJ. 1993. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 42, 265–292. ( 10.1093/sysbio/42.3.265) [DOI] [Google Scholar]
- 70.Freckleton RP. 2012. Fast likelihood calculations for comparative analyses. Methods Ecol. Evol. 3, 940–947. ( 10.1111/j.2041-210X.2012.00220.x) [DOI] [Google Scholar]
- 71.Blomberg SP, Garland T Jr, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. ( 10.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 72.Sereno PC, Larsson HC, Sidor CA, Gado B. 2001. The giant crocodyliform Sarcosuchus from the Cretaceous of Africa. Science 294, 1516–1519. ( 10.1126/science.1066521) [DOI] [PubMed] [Google Scholar]
- 73.Buscalioni AD, Piras P, Vullo R, Signore M, Barbera C. 2011. Early eusuchia crocodylomorpha from the vertebrate-rich Plattenkalk of Pietraroia (Lower Albian, southern Apennines, Italy). Zool. J. Linnean Soc. 163, S199–S227. ( 10.1111/j.1096-3642.2011.00718.x) [DOI] [Google Scholar]
- 74.Hua S, de Buffrénil V. 1996. Bone histology as a clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia). J. Vertebr. Paleontol. 16, 703–717. ( 10.1080/02724634.1996.10011359) [DOI] [Google Scholar]
- 75.Bickler PE, Buck LT. 2007. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu. Rev. Physiol. 69, 145–170. ( 10.1146/annurev.physiol.69.031905.162529) [DOI] [PubMed] [Google Scholar]
- 76.Brix O, Condo SG, Lazzarino G, Clémenti ME, Scatena R, Giardina B. 1989. Arctic life adaptation-III. The function of whale (Balaenoptera acutorastrata) hemoglobin. Comp. Biochem. Physiol. 94B, 139–142. ( 10.1016/0305-0491(89)90024-2) [DOI] [PubMed] [Google Scholar]
- 77.Lestyk KC, Folkow LP, Blix AS, Hammil MO, Burns JM. 2009. Development of myoglobin concentration and acid buffering capacity in harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals from birth to maturity. J. Comp. Physiol. B 179, 985–996. ( 10.1007/s00360-009-0378-9) [DOI] [PubMed] [Google Scholar]
- 78.Andrews RD, Enstipp MR. 2016. Diving physiology of seabirds and marine mammals: relevance, challenges and some solutions for field studies. Comp. Biochem. Physiol. A 202, 38–52. ( 10.1016/j.cbpa.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 79.Artioli GG, Gualano B, Smith A, Stout J, Lancha AH Jr. 2010. The role of β-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc. 42, 1162–1173. ( 10.1249/MSS.0b013e3181c74e38) [DOI] [PubMed] [Google Scholar]
- 80.Baguet A, Koppo K, Pottier A, Derave W. 2010. β-Alanine supplementation reduces acidosis but not oxygen uptake response during high-intensity cycling exercise. Eur. J. Appl. Physiol. 108, 495–503. ( 10.1007/s00421-009-1225-0) [DOI] [PubMed] [Google Scholar]
- 81.Boldyrev AA, Aldini G, Derave W. 2013. Physiology and pathophysiology of carnosine. Physiol. Rev. 93, 1803–1845. ( 10.1152/physrev.00039.2012) [DOI] [PubMed] [Google Scholar]
- 82.Clarac F, Souter T, Cubo J, de Buffrénil V, Brochu C, Cornette R. 2016. Does skull morphology constrain bone ornamentation? A morphometric analysis in the Crocodylia. J. Anat. 229, 292–301. ( 10.1111/joa.12470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lutz PL, Musick JA, Wyneken J. 2002. The biology of the sea turtles, vol. 2. Boca Raton, FL: CRC Press. [Google Scholar]
- 84.Broderick AC, Godley BJ, Hays GC. 2001. Trophic status drives interannual variability in nesting numbers of marine turtles. Proc. R. Soc. Lond. B 268, 1481–1487. ( 10.1098/rspb.2001.1695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hochscheid S, Bentivegna F, Speakman JR. 2003. The dual function of the lung in chelonian sea turtles: buoyancy control and oxygen storage. J. Exp. Mar. Biol. Ecol. 297, 123–140. ( 10.1016/j.jembe.2003.07.004) [DOI] [Google Scholar]
- 86.Hochscheid S, McMahon CR, Bradshaw CJA, Maffucci F, Bentivegna F, Hays GC. 2007. Allometric scaling of lung volume and its consequences for marine turtle diving performance. Comp. Biochem. Phys. A 148, 360–367. ( 10.1016/j.cbpa.2007.05.010) [DOI] [PubMed] [Google Scholar]
- 87.Dinkelacker SA, Costanzo JP, Lee RE Jr. 2005. Anoxia tolerance and freeze tolerance in hatchling turtles. J. Comp. Physiol. B 175, 209–217. ( 10.1007/s00360-005-0478-0) [DOI] [PubMed] [Google Scholar]
- 88.Reese SA, Jackson DC, Ultsch GR. 2003. Hibernation in freshwater turtles: softshell turtles (Apalone spinifera) are the most intolerant of anoxia among North American species. J. Comp. Physiol. B 173, 263–268. ( 10.1007/s00360-003-0332-1) [DOI] [PubMed] [Google Scholar]
- 89.Dunson WA. 1960. Aquatic respiration in Trionyx spinifer asper. Herpetologica 16, 277–283. [Google Scholar]
- 90.Feder ME, Burggren WW. 1985. Cutaneous gas exchange in vertebrates: design, patterns, control and implications. Biol. Rev. 60, 1–45. ( 10.1111/j.1469-185X.1985.tb00416.x) [DOI] [PubMed] [Google Scholar]
- 91.Hochscheid S, Bentivegna F, Speakman JR. 2002. Regional blood flow in sea turtles: implications for heat exchange in an aquatic ectotherm. Physiol. Biochem. Zool. 75, 66–76. ( 10.1086/339050) [DOI] [PubMed] [Google Scholar]
- 92.Kumazawa Y, Nishida M. 1999. Complete mitochondrial DNA sequences of the green turtle and blue-tailed mole skink: statistical evidence for archosaurian affinity of turtles. Mol. Biol. Evol. 16, 784–792. ( 10.1093/oxfordjournals.molbev.a026163) [DOI] [PubMed] [Google Scholar]
- 93.Zardoya R, Meyer A. 1998. Complete mitochondrial genome suggests diapsid affinities of turtles. Proc. Natl Acad. Sci. USA 95, 14 226–14 231. ( 10.1073/pnas.95.24.14226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zardoya R, Meyer A. 2001. The evolutionary position of turtles revised. Naturwissenschaften 88, 193–200. ( 10.1007/s001140100228) [DOI] [PubMed] [Google Scholar]
- 95.Cao Y, Sorenson MD, Kumazawa Y, Mindell DP, Hasegawa M. 2000. Phylogenetic position of turtles among amniotes: evidence from mitochondrial and nuclear genes. Gene 259, 139–148. ( 10.1016/S0378-1119(00)00425-X) [DOI] [PubMed] [Google Scholar]
- 96.Hedges SB, Poling LL. 1999. A molecular phylogeny of reptiles. Science 283, 998–1001. ( 10.1126/science.283.5404.998) [DOI] [PubMed] [Google Scholar]
- 97.Crawford NG, Faircloth BC, McCormackn JE, Brumfield RT, Winker K, Travis C, Glenn TC. 2012. More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biol. Lett. 8, 783–786. ( 10.5061/dryad.75nv22qj) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joyce WG. 2015. The origin of turtles: a paleontological perspective. J. Exp. Zool. B Mol. Dev. Evol. 324B, 181–193. ( 10.1002/jez.b.22609) [DOI] [PubMed] [Google Scholar]
- 99.Scheyer TM, Danilov IG, Sukhanov VB, Syromyatnikova EV. 2014. The shell bone histology of fossil and extant marine turtle revisited. Biol. J. Linn. Soc. 112, 701–718. ( 10.1111/bij.12265) [DOI] [Google Scholar]
- 100.Ricqlès A, Padian K, Horner JR. 2003. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Ann. Paleontol. 89, 67–101. ( 10.1016/S0753-3969(03)00005-3) [DOI] [Google Scholar]
- 101.Ricqlès A, Padian K, Knoll F, Horner JR. 2008. On the origin of high growth rates in archosaurs and their ancient relatives: complementary histological studies on Triassic archosauriforms and the problem of a ‘phylogenetic signal’ in bone histology. Ann. Paleontol. 94, 57–76. ( 10.1016/j.annpal.2008.03.002) [DOI] [Google Scholar]
- 102.Seymour RS, Bennett-Stamper CL, Johnston SD, Carrier DR, Grigg GC. 2004. Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. Physiol. Biochem. Zool. 77, 1051–1067. ( 10.1086/422766) [DOI] [PubMed] [Google Scholar]
- 103.Legendre L, Segalen L, Cubo J. 2013. Evidence for high bone growth rate in Euparkeria obtained using a new paleohistological inference model for the humerus. J. Vertebr. Paleontol. 33, 1343–1350. ( 10.1080/02724634.2013.780060) [DOI] [Google Scholar]
- 104.Legendre L, Guenard G, Botha-Brink J, Cubo J. 2016. Palaeohistological evidence for ancestral high metabolic rate in archosaurs. Syst. Biol. 65, 989–996. ( 10.1093/sysbio/syw033) [DOI] [PubMed] [Google Scholar]
- 105.Lyson TR, Rubidge B, Scheyer TM, de Queiroz K, Schachner ER, Smith RM, Botha-Brink J, Bever GS. 2016. Fossorial origin of the turtle shell . Curr. Biol. 14, 1887–1894. ( 10.1016/j.cub.2016.05.020) [DOI] [PubMed] [Google Scholar]
- 106.Seilacher A. 1970. Arbeitskonzept zur Konstruktionsmorphologie. Lethaia 3, 393–396. ( 10.1111/j.1502-3931.1970.tb00830.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are accessible in electronic supplementary material files.