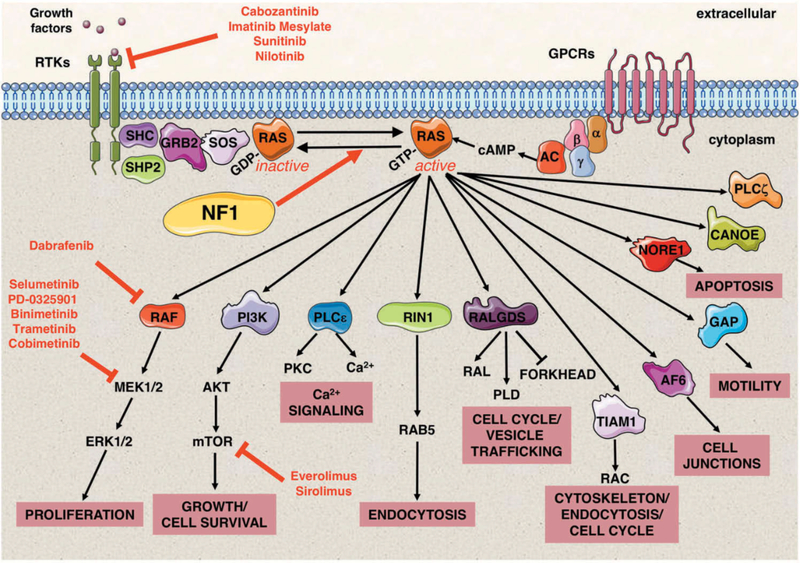
Figure 2. Neurofibromin is a negative regulator of the RAS signaling pathway.
RAS proteins function as fundamental signaling switches controlling a multitude of cellular processes including normal cell growth and differentiation. RAS proteins exist in two cellular states: an inactive GDP-bound form and an active GTP-bound form. Although RAS has a low intrinsic GTPase-activity, RAS-GTPase activating proteins (GAPs) stimulate this activity and consequently help to hydrolyze bound GTP back to GDP. Conversely, guanine nucleotide exchange factors (GEFs), e.g. SOS, convert RAS to its active GTP-bound form. NF1 encodes neurofibromin, a RAS-GAP, which is able to negatively regulate HRAS, KRAS, NRAS, MRAS, RRAS, and RRAS2 (TC21). Neurofibromin deficiency therefore results in elevated RAS signaling. RAS signaling transduces extracellular signals from ligand-activated receptors, both receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs) at the cell surface through multiple pathways. RAS-GTP proteins activate a multitude of effector proteins, including RAF proteins (ARAF, BRAF, and CRAF) and the MEK–ERK signaling cascade, RalGDS, and the PI3 kinase family. The molecular and cellular events controlled by normal and aberrantly regulated RAS signaling are cell-type specific, depending on the expression of different RAS isoforms and their relative engagement of particular RAS effector proteins. Small molecule inhibitors to upstream regulators of RAS and its downstream effectors in NF1 clinical trials are shown in red. Abbreviations: Growth factor receptor-bound protein 2 (GRB2), src homology 2 domain (SHC), adenyl cyclase (AC), cyclic adenosine monophosphate (cAMP), α, β and γ represent the subunits of a heterotrimeric G protein complex.