Table 1. Optimization studies a .
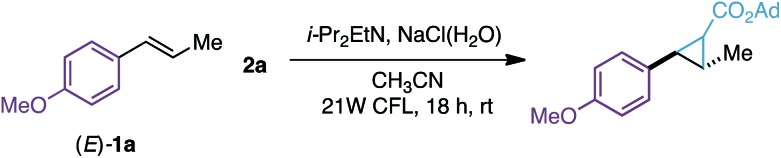
| ||
| Entry | Deviation of the reaction conditions | Yield b 3a, % |
| 1 | None | 91 c (75) d |
| 2 | Without NaCl (H2O) | 17 |
| 3 | Without NaCl | 48 |
| 4 | Under air | 10 |
| 5 | Without i-Pr2EtN | 0 |
| 6 | In the dark | 0 |
aReaction conditions: 1a (0.10 mmol), 2a (0.10 mmol), i-PrEt2N (0.20 mmol), CH3CN (1 mL), NaCl (1.25 M in H2O, 0.5 mL). Reactions were degassed prior to irradiation.
b 1H-NMR yields calculated using 1,2-dimethoxyethane as the internal standard.
cYield of the isolated product adding additional 2a (0.10 mmol, 1 equiv.) and i-PrEt2N (0.20 mmol, 2 equiv.) after 4 hours.
dYield of the isolated product using 1 gram of (E)-1a and 1 equiv. of 2a. See the ESI for the evaluation of other solvents, amines, or visible-light sources.
