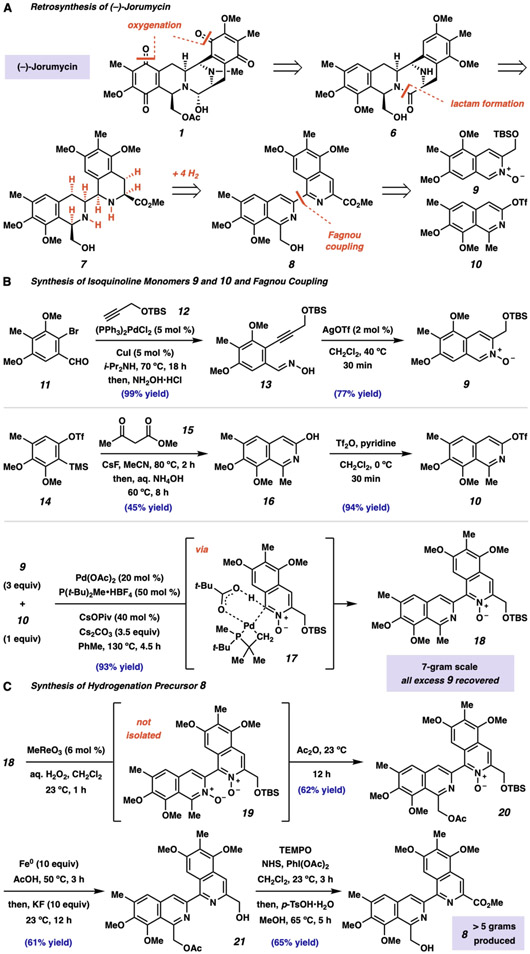
Fig. 2. Considerations for an orthogonal synthesis of jorunnamycin A and jorumycin.
(A) Retrosynthetic analysis leading to a synthesis of jorumycin that deviates from previous synthetic strategies. (B) Isoquinoline 9 and 10 were synthesized in two steps each from aryl bromide 11 and ortho-silyl aryl triflate 14, respectively. (C) Boekelheide rearrangement provided an efficient and scalable route to bis-isoquinoline 8 under mild conditions. TBS, tert-butyldimethylsilyl; Ph, phenyl; i-Pr, isopropyl; aq., aqueous; Tf, trifluoromethanesulfonyl, TMS, trimethylsilyl; MeCN, acetonitrile; t-Bu, tert-butyl; Piv, trimethyl-acetyl; equiv, molar equivalent; TEMPO, 2,2,6,6-tetramethylpiperidine-N-oxyl; NHS, N-hydroxysuccinimide; p-TsOH•H2O, para-toluenesulfonic acid monohydrate.