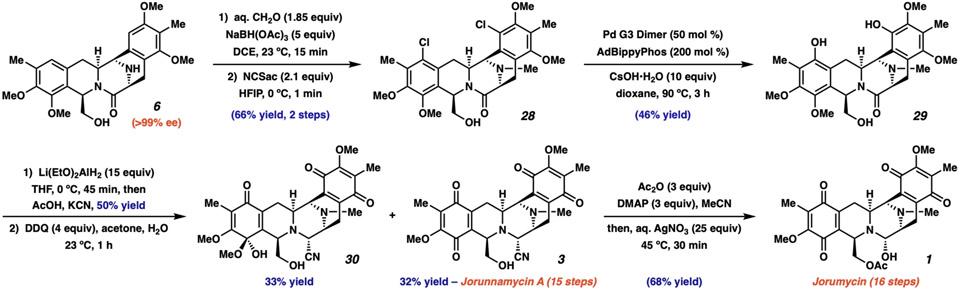
Fig. 4. Completion of jorunnamycin A and jorumycin.
After the reductive cyclization, five and six steps, including a palladium-catalyzed hydroxylation event, were required for the complete synthesis of jorunnamycin A (3) and jorumycin (1), respectively. DCE, 1,2-dichloroethane; NCSac, N-chlorosaccharine; HFIP, 1,1,1,3,3,3-hexafluoroisopropanol; Ad, 1-adamantyl; Pd G3 Dimer, (2'-Amino-1,1'-biphenyl-2-yl)methanesulfonatopalladium(II) dimer; DDQ, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; DMAP, 4-dimethylaminopyridine.