Abstract
Study Objectives
Determine the association of poor multidimensional sleep health with health-care costs and utilization.
Methods
We linked 1,459 community-dwelling women (mean age 83.6 years) participating in the Study of Osteoporotic Fractures Year 16 visit (2002–2004) with their Medicare claims. Five dimensions of sleep health (satisfaction, daytime sleepiness, timing, latency, and duration) were assessed by self-report. The number of impaired dimensions was expressed as a score (range 0–5). Total direct health-care costs and utilization were ascertained during the subsequent 36 months.
Results
Mean (SD) total health-care costs/year (2017 dollars) increased in a graded manner across the sleep health score ranging from $10,745 ($15,795) among women with no impairment to up to $15,332 ($22,810) in women with impairment in three to five dimensions (p = 0.01). After adjustment for age, race, and enrollment site, women with impairment in three to five dimensions vs. no impairment had greater mean total costs (cost ratio [CR] 1.34 [95% CI = 1.13 to 1.60]) and appeared to be at higher risk of hospitalization (odds ratio (OR) 1.31 [95% CI = 0.96 to 1.81]). After further accounting for number of medical conditions, functional limitations, and depressive symptoms, impairment in three to five sleep health dimensions was not associated with total costs (CR 1.02 [95% CI = 0.86 to 1.22]) or hospitalization (OR 0.91 [95% CI = 0.65 to 1.28]). Poor multidimensional sleep health was not related to outpatient costs or risk of skilled nursing facility stay.
Conclusions
Older women with poor sleep health have higher subsequent total health-care costs largely attributable to their greater burden of medical conditions, functional limitations, and depressive symptoms.
Keywords: aging, sleep health, health-care costs, health-care utilization
Statement of Significance.
Previous studies examining the association of sleep with health-care costs and utilization have primarily focused on specific sleep disorders such as insomnia and sleep apnea. Most have not been restricted to older adults, where sleep complaints are most common and health-care expenditures are highest. We used a unique longitudinal dataset of community-dwelling women in the ninth decade of life linked with their Medicare claims data to determine the association of a multidimensional measure of poor subjective sleep health with subsequent total health-care costs and utilization. We found that older women with poor sleep health have higher subsequent total health-care costs largely attributable to their greater burden of medical conditions, functional limitations, and depressive symptoms.
Introduction
Sleep complaints, including difficulty falling asleep, frequent awakening, awaking too early, daytime sleepiness, and not feeling rested, are increasingly common with advancing age [1, 2]. Characteristics indicative of “poor sleep” are associated with a variety of age-related diseases and adverse clinical outcomes, including depression [3–5], diabetes [6], cardiovascular disease [7–10], and increased risks of falls [11–13] and mortality [14–18]. Sleep complaints in older adults are also related to the presence of multiple chronic medical conditions (multimorbidity) [19] and functional limitations [20, 21]. These linkages suggest that poor sleep may be associated with higher health-care expenditures in the aged population.
Previous studies examining the association of sleep with health-care costs and utilization have primarily focused on specific sleep disorders such as insomnia and sleep apnea [22–28]. Most investigations have not been restricted to older adults, where sleep complaints, medical conditions, and functional impairment are most common and health-care expenditures are highest.
Sleep can be measured across multiple dimensions of sleep health, including satisfaction/quality, alertness/sleepiness, timing, continuity or efficiency, and duration. These dimensions of sleep health are not specific to any individual sleep disorder and can be assessed by self-report [29, 30]. Considering sleep as a multidimensional construct, rather than a series of separate sleep characteristics or specific sleep disorders, may provide a more comprehensive assessment of overall sleep health.
To examine the association of a multidimensional measure of poor subjective sleep health with subsequent total health-care costs and utilization, we used a unique longitudinal dataset comprising 1,459 women (mean age 83.6 years) participating in the Year 16 (Y16) visit of the Study of Osteoporotic Fractures (SOF) linked with their Medicare claim data.
Methods
Study population and linkage to Medicare claims
We studied participants enrolled in SOF, a prospective cohort study of community-dwelling women. At the baseline visit conducted between 1986 and 1988, 9,704 Caucasian women of at least 65 years old and able to walk unassisted were recruited for participation from four geographic areas of the United States [31]. Initially African Americans were excluded from SOF due to their low incidence of hip fracture, but an additional 662 African American women of at least 65 years were enrolled in SOF between 1997 and 1998 bringing the total enrollment to 10,366 women.
Linkage of the SOF cohort data and Medicare claims was successful for 9,228 women (92.3 % of surviving participants) as of January 1, 1991, the earliest data for which outpatient Medicare claims are available [32, 33]. There were 3,563 women from the cohort who participated in the Y16 visit between 2002 and 2004 and had complete data on self-reported sleep dimensions, functional limitations, chronic medical conditions, and depressive symptoms at this time point (Supplementary Figure S1). Of these women, the analytic cohort included 1,459 women who were also enrolled in the Medicare Fee-For-Service (FFS) program (Parts A and B [and not Part C, Medicare Advantage]) at the Y16 visit until 36 months following this date (or up until death within this period).
Assessment of sleep health dimensions
Each participant at the Y16 visit was asked the following questions about current sleep patterns during the past month: on most nights, how many hours do you sleep; how many hours of sleep do you need to feel rested; at what time do you usually fall asleep; at what time do you usually wake up; and how long does it usually take you to fall asleep each night. Participants also completed the Epworth Sleepiness Scale (ESS), a self-administered questionnaire that assesses daytime sleepiness [34, 35].
Women for whom hours of sleep needed to feel rested exceeded average hours slept at night were scored as “poor” in the sleep satisfaction/quality dimension. Women with an ESS score of more than 10 (the threshold for excessive daytime sleepiness) were classified as “poor” in the alertness/sleepiness dimension [34, 35]. Mid-sleep time was assessed by identifying the clock time halfway between falling asleep time and waking up time and was calculated as the sum of falling asleep time + (waking up time − falling asleep time)/2. Mid-sleep time was categorized based on octiles of the mid-sleep time; women in the first (e.g. earlier midpoint of sleep time) or eighth (e.g. later midpoint of sleep time) octile were classified as “poor” in the dimension of sleep timing. Women who responded that it took 30 minutes or more to fall asleep were classified as “poor” in sleep onset latency dimension. Women who reported sleeping less than 7 hours or 9 hours or more were classified as “poor” in the sleep duration dimension.
A multidimensional measure of poor sleep health (range 0–5) was calculated by summing the number of dimensions with poor scores and categorized as 0, 1, 2, or at least 3 impairments in sleep health dimensions.
Outcome measures
The primary outcome was total direct health-care costs/year for the 36 months following the Y16 visit. For the 196 women (13.4%) who died within the 36-month period, total health-care costs were calculated from the date of the Y16 visit until the date of death. Total costs were calculated as the sum of costs for acute hospital stays, skilled nursing facility (SNF) stays paid under Medicare Part A, inpatient rehabilitation facility (IRF) stays, outpatient care, and home health care for that time period. Acute hospital stays, SNF stays, and IRF stays were identified in the Medical Provider Analysis and Review (MedPAR) file. Standardized costs for acute short hospital stays, SNF stays, and IRF stays were estimated using a previously published and validated method [33, 36]. Costs for outpatient utilization and home health-care utilization were based on the allowable charges for these services in the Carrier, Outpatient, and Home Health Care Medicare claims files [37]. The costs of all units of utilization were adjusted for health-care cost inflation to US 2017 dollars [33].
Other measurements
Each participant completed a questionnaire and was asked at the Y16 visit about smoking status, alcohol use, caffeine intake, and whether she lived alone. Women were asked about a physician diagnosis of hypertension, myocardial infarction, angina, congestive heart failure, stroke, diabetes, chronic obstructive pulmonary disease/asthma, Alzheimer’s disease/other dementia, depression, cancer, osteoarthritis, rheumatoid arthritis, osteoporosis, and Parkinsonism. A summary multimorbidity score (range 0–14) was then created and categorized as none, 1, 2, 3, or at least 4 of these medical conditions. Women were also asked about a physician diagnosis of sleep apnea and sleep disorder other than sleep apnea including insomnia, narcolepsy, and restless leg syndrome. Women were asked whether they had any difficulty performing each of five instrumental activities of daily living (IADL; walking 2 or 3 blocks, climbing 10 steps without stopping, preparing one’s own meals, doing heavy housework, shopping for one’s own groceries or clothes) and each of three basic activities of daily living (ADL; dressing yourself, getting in or out of bed, or washing and drying entire body). Functional limitations were categorized as no, 1, 2, 3, or at least 4 difficulties in IADL and none vs. at least 1 difficulty with basic ADL. Depressive symptoms were assessed using the Geriatric Depression Scale (GDS) [38] and categorized as none or minimal (GDS score 0–1), mild (GDS score 2–5), or moderate to severe (GDS score ≥6). The Mini-Mental State Examination [39] was administered (range 0–30). Women were asked to bring all drug containers used within the previous 30 days with them to the Y16 visit. Drugs were identified and recorded by clinic staff. All medications recorded by the clinic staff were stored in an electronic medications inventory database at the study data coordinating center; a computerized dictionary was used to categorize type of medication from product brand and generic names obtained from containers [40]. Information from the earlier SOF visits was used to assess self-reported race/ethnicity.
Statistical analysis
Generalized linear models (GLMs) were used to estimate the association of the multidimensional measure of poor sleep health with mean annualized total direct health-care costs over the subsequent 36 months. Gamma distributions with log links were chosen based on Modified Park [41] and Pregibon link tests [42]. GLMs (gamma distributions, log links) were also used to analyze the association of the multidimensional measure of poor sleep health with mean annualized outpatient costs. Logistic models were used to estimate the associations of the multidimensional measure of poor sleep health with risks of at least 1 hospitalization and at least 1 SNF stay. Women with no impairment in any sleep health dimension were the referent group. In addition, GLMs (gamma distributions, log links) were used to determine the association of each individual dimension of poor sleep health with subsequent total health-care costs/year.
Base models included age, race, and enrollment site. To determine if any association of the multidimensional poor sleep health score with health-care costs and utilization outcomes was explained by greater multimorbidity, limitations in performing IADL and ADL, or depressive symptoms among those with poorer sleep, we added these variables (one at a time) to the base models. Finally, base models were further simultaneously adjusted for all these potential confounders.
A sensitivity analyses was performed for the primary outcome of total health-care costs excluding the 196 women who died during the 36-month follow-up period. In addition, to examine whether any association between poor multidimensional sleep health and total health-care costs was due to the effects of diagnosed sleep disorders or use of hypnotic medications, we performed an analysis excluding the 243 women (16.7%) who reported a physician diagnosis of sleep apnea or other sleep disorder or those reporting current use of benzodiazepines or non-benzodiazepine sedative hypnotic medications.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and all significance levels reported were two sided.
Results
The study cohort at Y16 included 1,459 community-dwelling women with a mean age of 83.6 (3.9) years; 173 women (11.9%) reported African American race (Table 1). Among the cohort, 310 women (21.2%) had no impairment in any sleep health dimension, 456 (31.3%) had impairment in one dimension, 355 (24.3%) had impairment in two dimensions, and 338 (23.2%) had impairment in three to five 5 dimensions. Self-reported functional limitations, multimorbidity, and depressive symptoms were common. There were 733 women (50.2%) who reported difficulty performing at least one IADL and 234 (16.0%) with difficulty performing at least one ADL. Similarly, 1,000 women (68.5%) had two or more medical conditions and 781 (53.5%) had at least mild (GDS score ≥2) depressive symptoms. Women with poor sleep health as manifested by more impairments in sleep health dimensions had a greater number of functional limitations, coexisting medical conditions and depressive symptoms. In addition, women with poor multidimensional sleep health were more likely to be African American.
Table 1.
Characteristics of overall cohort and by number of impairments in sleep health dimensions
| Characteristics | Number of impairments in sleep health dimensions | |||||
|---|---|---|---|---|---|---|
| Overall cohort | 0 | 1 | 2 | 3–5 | ||
| (n = 1,459) | (n = 310) | (n = 456) | (n = 355) | (n = 338) | P-value | |
| Age, years, mean (SD) | 83.6 (3.90) | 83.5 (3.92) | 83.9 (4.11) | 83.7 (3.75) | 83.3 (3.76) | 0.17 |
| African American, n (%) | 173 (11.9) | 20 (6.5) | 51 (11.2) | 46 (13.0) | 56 (16.6) | <0.001 |
| Lives alone, n (%) | 875 (60.0) | 181 (58.4) | 279 (61.2) | 214 (60.3) | 201 (59.5) | 0.88 |
| Ever smoker, n (%) | 543 (41.8) | 135 (46.4) | 157 (38.3) | 134 (43.1) | 117 (40.9) | 0.18 |
| Alcoholic drinks/week past 30 days, mean (SD) | 1.3 (3.22) | 1.7 (3.46) | 1.3 (3.13) | 1.1 (3.15) | 1.2 (3.17) | 0.08 |
| Caffeine intake, mg/d, mean (SD) | 137 (144) | 144 (145) | 136 (143) | 135 (147) | 132 (140) | 0.78 |
| No. of IADL impairment, n (%) | <0.001 | |||||
| None | 726 (49.8) | 190 (61.3) | 226 (49.6) | 172 (48.5) | 138 (40.8) | |
| 1 | 241 (16.5) | 42 (13.5) | 80 (17.5) | 62 (17.5) | 57 (16.9) | |
| 2 | 176 (12.1) | 34 (11.0) | 50 (11.0) | 41 (11.5) | 51 (15.1) | |
| 3 | 135 (9.3) | 20 (6.5) | 48 (10.5) | 26 (7.3) | 41 (12.1) | |
| ≥4 | 181 (12.4) | 24 (7.7) | 52 (11.4) | 54 (15.2) | 51 (15.1) | |
| No. of ADL impairment, n (%) | <0.001 | |||||
| None | 1225 (84.0) | 279 (90.0) | 391 (85.7) | 289 (81.4) | 266 (78.7) | |
| 1 or more | 234 (16.0) | 31 (10.0) | 65 (14.3) | 66 (18.6) | 72 (21.3) | |
| Geriatric Depression Scale score, n (%) | <0.001 | |||||
| 0–1 | 678 (46.5) | 187 (60.3) | 235 (51.5) | 148 (41.7) | 108 (32.0) | |
| 2–5 | 602 (41.3) | 107 (34.5) | 173 (37.9) | 157 (44.2) | 165 (48.8) | |
| ≥6 | 179 (12.3) | 16 (5.2) | 48 (10.5) | 50 (14.1) | 65 (19.2) | |
| Medical conditions* (0–14), n (%) | <0.001 | |||||
| None | 127 (8.7) | 37 (11.9) | 48 (10.5) | 28 (7.9) | 14 (4.1) | |
| 1 | 332 (22.8) | 88 (28.4) | 105 (23.0) | 78 (22.0) | 61 (18.0) | |
| 2 | 353 (24.2) | 78 (25.2) | 117 (25.7) | 88 (24.8) | 70 (20.7) | |
| 3 | 325 (22.3) | 61 (19.7) | 100 (21.9) | 75 (21.1) | 89 (26.3) | |
| ≥4 | 322 (22.1) | 46 (14.8) | 86 (18.9) | 86 (24.2) | 104 (30.8) | |
| MMSE score, mean (SD) | 27.6 (2.04) | 27.6 (2.14) | 27.5 (2.00) | 27.5 (2.07) | 27.7 (1.95) | 0.35 |
| Hospitalization in past year, n (%) | 354 (24.3) | 67 (21.6) | 112 (24.6) | 78 (22.0) | 97 (28.7) | 0.12 |
| Incident hospitalization, n (%) | 851 (58.3) | 164 (52.9) | 259 (56.8) | 221 (62.3) | 207 (61.2) | 0.05 |
| Incident SNF stay, n (%) | 355 (24.3) | 76 (24.5) | 100 (21.9) | 88 (24.8) | 91 (26.9) | 0.44 |
| Total annual health-care costs, US $ | ||||||
| Median (IQR) | $6,318 | $4,987 | $6,387 | $6,811 | $7,267 | |
| (2,297–16,086) | (1,751–13,423) | (2,411–15,703) | (2,564–16,470) | (2,473–17,572) | ||
| Mean (SD) | $12,588 (18,057) | $10,745 (15,795) | $11,773 (16,151) | $12,632 (16,837) | $15,332 (22,810) | 0.007 |
| Annual outpatient costs, US $ | ||||||
| Median (IQR) | $2,680 | $2,523 | $2,710 | $2,813 | $2,868 | |
| (1,506–4,733) | (1,281–4,065) | (1,553–4,634) | (1,448–4,925) | (1,661–5,009) | ||
| Mean (SD) | $3,808 (4,236) | $3,553 (4,364) | $3,961 (4,892) | $3,725 (3,402) | $3,925 (3,948) | 0.55 |
| Died during follow-up, n (%) | 196 (13.4) | 28 (9.0) | 58 (12.7) | 58 (16.3) | 52 (15.4) | 0.03 |
IQR, interquartile range; MMSE, Mini-Mental State Examination.
*Medical conditions include hypertension, congestive heart failure, myocardial infarction, angina, stroke, diabetes, chronic lung disease, cancer, osteoarthritis, rheumatoid arthritis, depression, Alzheimer’s disease, parkinsonism, and osteoporosis.
Among the overall cohort during the 36 months following Y16, the annualized median (interquartile range) total health-care costs (2017 US dollars) were $6,318 ($2,297–$16,086) and annualized mean (SD) costs were $12,588 ($18,057). During this time period, 851 women (58.3%) had at least one hospitalization and 355 (24.3%) had at least one SNF stay.
Characteristics (including the distribution of the number of impairments in sleep health dimensions) of the 1,459 women in the analytical cohort were similar to those of the 2,104 SOF women attending Y16 visit who were excluded from analyses because they were not enrolled in a FFS plan (Supplementary Table S1). Although differences in race, smoking status, alcohol intake, caffeine consumption, functional limitations, and cognitive function were statistically significant, all of these differences were small in magnitude.
Association of multidimensional measure of poor sleep health with health-care costs
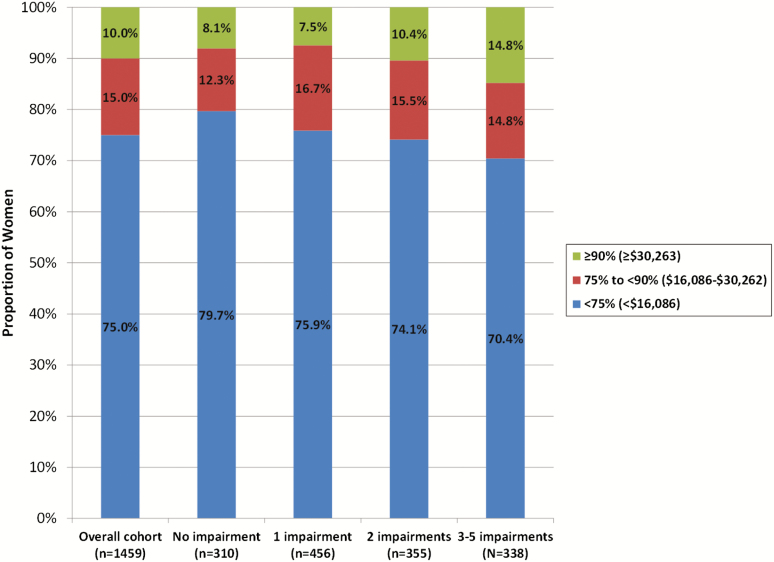
Annualized mean and median total health-care costs in the 36 months after Y16 visit were higher with greater number of impairments in sleep health dimensions at Y16 (Table 1). Mean (SD) costs increased in a graded manner from $10,745 ($15,795) among women with no impairment up to $15,332 ($22,810) among women with three to five impairments (p = 0.007). Greater number of impairments in sleep health dimensions was also associated with a higher likelihood of incurring very high costs; the proportion of women with costs in the highest decile ($30,263–$237,480) was 8.1% among women with no impairments increasing to 14.8% among women with three to five impairments (p = 0.01) (Figure 1).
Figure 1.
Distribution of total health-care costs overall and according to number of impairments in sleep health dimensions.
After adjustment for age, race, and enrollment site, mean total costs were 1.3-fold higher (cost ratio [CR] 1.34 [95% CI = 1.13 to 1.60]) among women with three to five impairments in sleep health dimensions vs. those with no impairments (Table 2). This association persisted (CR 1.37 [95% CI = 1.07 to 1.57]) when 243 women (16.7%) who reported a physician diagnosis of a sleep disorder or current use of benzodiazepines or non-benzodiazepine sedative hypnotic medications were excluded from the analysis. After further adjusting for functional limitations, number of chronic medical conditions, or depressive symptoms one at a time to the base model, the association was attenuated in magnitude to a similar degree and no longer significant. After simultaneous consideration of functional limitations, multimorbidity, and depressive symptoms, there was no association of poor multidimensional sleep health with total health-care costs (CR 1.02 [95% CI = 0.86 to 1.22]). In contrast, greater burden of medical conditions, IADL limitations and depressive symptoms were each independently associated with higher total health-care costs after accounting for each other and number of impairments in sleep health dimensions (Supplementary Table S2). Findings were similar in analyses limited to the 1,263 women who survived for at least 36 months after the Y16 visit.
Table 2.
Association of number of impairments in sleep health dimensions with mean total and outpatient health-care costs
| Total health-care costs | Outpatient costs | ||
|---|---|---|---|
| Impairments in sleep health dimensions | CR (95% CI) | P-value | CR (95% CI) |
| Base model* | 0.007 | ||
| None | Reference | Reference | |
| 1 impairment | 1.08 (0.92 to 1.28) | 1.11 (0.98 to 1.26) | |
| 2 impairments | 1.16 (0.98 to 1.38) | 1.06 (0.93 to 1.21) | |
| 3–5 impairments | 1.34 (1.13 to 1.60) | 1.11 (0.97 to 1.27) | |
| Base model + number of medical conditions | 0.35 | ||
| None | Reference | Reference | |
| 1 impairment | 0.99 (0.84 to 1.16) | 1.07 (0.95 to 1.20) | |
| 2 impairments | 1.03 (0.86 to 1.22) | 1.00 (0.88 to 1.13) | |
| 3–5 impairments | 1.14 (0.95 to 1.36) | 1.00 (0.88 to 1.14) | |
| Base model + functional limitations | 0.26 | ||
| None | Reference | Reference | |
| 1 impairment | 1.00 (0.85 to 1.17) | 1.04 (0.93 to 1.18) | |
| 2 impairments | 1.03 (0.87 to 1.21) | 0.99 (0.87 to 1.13) | |
| 3–5 impairments | 1.16 (0.97 to 1.37) | 1.02 (0.89 to 1.16) | |
| Base model + depressive symptoms | 0.33 | ||
| None | Reference | Reference | |
| 1 impairment | 1.08 (0.92 to 1.27) | 1.09 (0.97 to 1.23) | |
| 2 impairments | 1.06 (0.89 to 1.25) | 1.02 (0.90 to 1.16) | |
| 3–5 impairments | 1.18 (0.99 to 1.40) | 1.04 (0.91 to 1.19) | |
| Multivariate model† | 0.80 | ||
| None | Reference | Reference | |
| 1 impairment | 0.96 (0.82 to 1.13) | 1.03 (0.92 to 1.16) | |
| 2 impairments | 0.95 (0.80 to 1.12) | 0.96 (0.85 to 1.09) | |
| 3–5 impairments | 1.02 (0.86 to 1.22) | 0.95 (0.83 to 1.08) |
*Adjusted for age, race, and enrollment site.
†Adjusted for age, race, enrollment site, number of medical conditions, functional limitations, and depressive symptoms.
There was no evidence of an association of poor multidimensional sleep health with outpatient costs.
Association of multidimensional measure of poor sleep health with hospitalization and SNF stay
Women with poor multidimensional sleep health appeared to have an increased odds of subsequent hospitalization after accounting for age, race, and enrollment site (odds ratio (OR) 1.31 [95% CI = 0.96 to 1.81] for women with three to five impairments vs. no impairments in sleep health dimensions), but this association did not reach the level of significance. The relationship was substantially attenuated after individual or simultaneous consideration of functional limitations, multimorbidity, and depressive symptoms (OR in full multivariable model 0.91 [95% CI = 0.65 to 1.28]) (Table 3). There was no evidence of an association of poor sleep health with odds of subsequent post-acute care SNF stay.
Table 3.
Associations of number of impairments in sleep health dimensions with odds of hospitalization and SNF stays
| ≥1 Hospitalization | ≥1 SNF stay | |
|---|---|---|
| Impairments in sleep health dimensions | OR (95% CI) | OR (95% CI) |
| Base model* | ||
| None | Reference | Reference |
| 1 impairment | 1.11 (0.82 to 1.49) | 0.83 (0.59 to 1.19) |
| 2 impairments | 1.38 (1.01 to 1.89) | 1.00 (0.70 to 1.44) |
| 3–5 impairments | 1.31 (0.96 to 1.81) | 1.18 (0.82 to 1.70) |
| Base model + number of medical conditions | ||
| None | Reference | Reference |
| 1 impairment | 1.05 (0.77 to 1.42) | 0.80 (0.56 to 1.13) |
| 2 impairments | 1.23 (0.89 to 1.70) | 0.92 (0.64 to 1.33) |
| 3–5 impairments | 1.06 (0.76 to 1.47) | 1.05 (0.73 to 1.53) |
| Base model + functional limitations | ||
| None | Reference | Reference |
| 1 impairment | 1.02 (0.75 to 1.38) | 0.75 (0.52 to 1.07) |
| 2 impairments | 1.25 (0.90 to 1.72) | 0.86 (0.59 to 1.24) |
| 3–5 impairments | 1.09 (0.78 to 1.52) | 0.95 (0.65 to 1.39) |
| Base model + depressive symptoms | ||
| None | Reference | Reference |
| 1 impairment | 1.05 (0.78 to 1.41) | 0.77 (0.54 to 1.10) |
| 2 impairments | 1.24 (0.90 to 1.71) | 0.86 (0.59 to 1.24) |
| 3–5 impairments | 1.10 (0.79 to 1.52) | 0.94 (0.64 to 1.37) |
| Multivariate model† | ||
| None | Reference | Reference |
| 1 impairment | 0.99 (0.72 to 1.34) | 0.72 (0.50 to 1.04) |
| 2 impairments | 1.13 (0.81 to 1.57) | 0.79 (0.54 to 1.16) |
| 3–5 impairments | 0.91 (0.65 to 1.28) | 0.86 (0.58 to 1.26) |
*Adjusted for age, race, and enrollment site.
†Adjusted for age, race, enrollment site, number of medical conditions, functional limitations, and depressive symptoms.
Associations of individual dimensions of poor sleep health with total health-care costs
Daytime sleepiness (CR 1.23 [95% CI = 1.02 to 1.49]) and short or long sleep duration (CR 1.21 [95% CI = 1.07 to 1.35]) (but not poor sleep satisfaction, poor sleep timing, or longer sleep latency) were each associated with higher mean total costs in models accounting for age, race, and enrollment site (Table 4). These associations were no longer present after further accounting for functional limitations, multimorbidity, and depressive symptoms.
Table 4.
Association of individual impairments in sleep health domains with mean total health-care costs
| CR (95% CI) | ||
|---|---|---|
| Sleep health dimension | Base model* | Multivariate Model† |
| Poor sleep satisfaction (n = 452) | 1.09 (0.96 to 1.23) | 0.95 (0.84 to 1.08) |
| Daytime sleepiness (n = 151) | 1.23 (1.02 to 1.49) | 1.03 (0.85 to 1.24) |
| Poor sleep timing (n = 455) | 1.11 (0.98 to 1.26) | 1.00 (0.88 to 1.13) |
| Long sleep latency (n = 562) | 1.01 (0.90 to 1.14) | 0.91 (0.81 to 1.03) |
| Short or long sleep duration (n = 690) | 1.21 (1.07 to 1.35) | 1.09 (0.97 to 1.22) |
*Adjusted for age, race, and enrollment site.
†Adjusted for age, race, enrollment site, number of medical conditions, functional limitations, and depressive symptoms.
Discussion
In our cohort of community-dwelling women in the ninth decade of life, poor multidimensional subjective sleep health was associated with higher subsequent total health-care costs. Nearly 1 in 6 women with impairments in three to five sleep health dimensions compared with 1 in 13 women with no impairments were among the top 10% of individuals with respect to total health-care costs. The association of multidimensional sleep health with health-care costs in women late in life is largely attributable to the greater burden of existing medical conditions, functional limitations, and depressive symptoms among those with poor multidimensional sleep health. Poor multidimensional sleep health was not an independent predictor of hospitalization and was not related to outpatient costs or risk of post-acute care SNF stay.
Our findings are generally consistent with those of prior studies in predominantly younger populations that have suggested an association of self-reported sleep complaints with greater health-care utilization [24–27]. In a cross-sectional study of a representative sample of 12,643 Hungarian adults aged 18 years and older [26], individuals with vs. those without insomnia (as defined by a score ≥10 on the Athens Insomnia Scale) more frequently reported hospitalization in the past year. Similarly, an analysis of data collected in 6,440 adults aged 40 years and older enrolled in the Sleep Heart Health Study [24] found associations of self-reported sleep measures including insomnia symptoms, inadequate sleep time, and daytime sleepiness with an indirect measure of health-care utilization (a modified Chronic Disease Score calculated based on current medication use). However, neither study evaluated whether these associations were independent of other traditional indicators such as burden of coexisting medical conditions. An analysis of data collected in the Health and Retirement Study [25] of 14,355 adults aged 55 years and older reported that participants with multiple insomnia symptoms vs. no symptoms had a 1.3-fold higher odds of subsequent hospitalization (assessed by self-report) after accounting for differences in demographic characteristics, selected medical conditions, and depressive symptoms. The presence of multiple insomnia symptoms was not an independent predictor of nursing home stays or use of home health-care services. Finally, a previous analysis of this cohort of older women linked with administrative claims data [43] found that subjectively assessed poorer sleep quality (assessed using the Pittsburgh Sleep Quality Index), but not excessive daytime sleepiness (assessed using the ESS), was associated with a 1.3-fold higher odds of hospitalization. However, this association did not persist in multivariable models adjusted for conventional predictors of this outcome.
A paucity of studies has examined the association of self-reported sleep measures or an insomnia diagnosis with health-care expenditures in older adults. In a retrospective study of an insurance claims database between 1999 and 2003 27, direct health-care costs (based on inpatient, outpatient, pharmacy, and emergency department claims) assessed during the previous 6 months among patients aged 65 years and older were about $1,100 greater for those with a new diagnosis or treatment of insomnia (defined using International Classification of Diseases, Ninth Revision [ICD-9] diagnostic codes or pharmacy claims for an insomnia medication) compared to propensity-matched controls. Of note, pharmacy claims did not contain information about indication for medication use and 36% of the older patients classified with a new diagnosis of insomnia based on drug use were taking selected antidepressants (e.g. amitriptyline, mirtazapine, trazodone) that have multiple indications for use. In our cohort, the association of poor multidimensional sleep health with higher total health-care costs after adjustment for demographic characteristics persisted in analyses excluding women who reported a physician diagnosis of a sleep disorder or current use of benzodiazepine or non-benzodiazepine sedative hypnotic medications. These findings suggest that the association of poor multidimensional sleep health with health-care expenditures is not confined to those individuals with recognized sleep disorders or deficits.
Our study expands upon these previously published studies with its focus on multidimensional sleep health, linkage with claims data to prospectively calculate health-care costs and confirm incident hospitalizations and SNF stays, and consideration of functional limitations, multimorbidity, and depressive symptoms as key potential confounding factors of the associations of poor sleep health with health-care use and expenditures. The increase in health-care costs among older women with impairments in three to five dimensions of sleep heath observed in our study was explained by greater functional limitations, multimorbidity, and depressive symptoms among this group. In contrast, we found that these characteristics were independently associated with higher health-care costs after consideration of each other and multidimensional sleep health. Thus, poor multidimensional sleep health may be a marker of these underlying characteristics and conditions that are associated with higher health-care expenditures. On the other hand, it is also possible that these factors are mediators of the association as poor sleep may worsen or exacerbate functional limitations, existing medical conditions, or depressive symptoms. A prior study in this cohort [44] reported that among women without clinically significant depressive symptoms (defined by a GDS score <6) at a Year 10 visit, those with more impairments in sleep health dimensions (assessed using similar, but not identical, criteria as in this study) vs. those with no impairments were more likely to have clinically significant depressive symptoms (i.e. GDS score ≥6) at the follow-up Y16 visit. However, this analysis did not account for multimorbidity, functional limitations, or GDS score at baseline. Future studies are needed to determine whether poor multidimensional sleep health is associated with subsequent decline in functional status or incident or worsening comorbid medical conditions. Of note, assessment of sleep health is not usually included in geriatric assessment or case management programs for older patients with multimorbidity. Thus, future randomized trials may be warranted to determine whether sleep health assessment and incorporation of sleep interventions (e.g. cognitive behavioral therapy for insomnia symptoms) into chronic disease management programs in high risk older adults reduces subsequent health-care utilization and costs.
Our study has a number of strengths including the comprehensive assessment of sleep health in each of the five dimensions, well characterized cohort representative of community-dwelling women later in life, linkage to inpatient claims data, and consideration of traditional robust predictors of health-care costs and utilization in older adults. However, this study has a several limitations. Results may not be generalizable to other populations including men, older adults residing in institutions and younger adults. Cost and utilization data were limited to women enrolled in FFS plans, although characteristics of women who were excluded based on not participating in FFS were similar to characteristics of included women. Furthermore, evidence suggests Medicare FFS and Medicare Advantage enrollees had similar health-care expenditures during the time period of this study [45]. While we assessed each of the five dimensions of sleep health, questions asked of participants were not identical to those proposed for the SATED scale measure of multidimensional sleep health [30]. Our measure of multidimensional sleep health equally weighted each sleep health dimension when it may be the case that specific dimensions affect health-care expenditures and use more strongly than others. In addition, the dimensions of sleep health were assessed by self-report, and future studies should evaluate the impact of objective measurements of poor sleep health with subsequent heath care costs. Finally, our study has an observational design. Thus, the possibility of residual confounding cannot be eliminated.
In conclusion, poor multidimensional subjective sleep health in this study of community-dwelling women in the ninth decade of life was associated with higher subsequent total health-care costs. This relationship was explained in large part by the greater burden of existing medical conditions, functional limitations, and depressive symptoms among women with poor multidimensional sleep health. These results underscore the complex and overlapping relationships between poor sleep health, multimorbidity, functional limitations, and depressive symptoms in the aged population.
Supplementary Material
Funding
This manuscript was supported with research grant support from Merck & Co. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576. This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Conflict of interest statement. Dr. Sonia Ancoli-Israel is a consultant to Merck, Eisai, Eli Lilly, and GlaxoSmithKline.
References
- 1. Foley DJ, et al. . Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. [DOI] [PubMed] [Google Scholar]
- 2. Newman AB, et al. . Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Morphy H, et al. . Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30(3):274–280. [PubMed] [Google Scholar]
- 4. Paudel ML, et al. ; Osteoporotic Fractures in Men Study Group. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56(7):1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smagula SF, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group. Inflammation, sleep disturbances, and depressed mood among community-dwelling older men. J Psychosom Res. 2014;76(5):368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gottlieb DJ, et al. . Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–867. [DOI] [PubMed] [Google Scholar]
- 7. Javaheri S, et al. ; Osteoporotic Fractures in Men Study Research Group. Sleep-disordered breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2016;193(5):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koo BB, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: Outcomes of Sleep Disorders in Older Men (MrOS) Study. Circulation. 2011;124(11):1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paudel ML, et al. . Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int. 2011;28(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stone KL, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39(3):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stone KL, et al. . Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168(16):1768–1775. [DOI] [PubMed] [Google Scholar]
- 12. Stone KL, et al. . Self-reported sleep and nap habits and risk of falls and fractures in older women: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 2006;54(8):1177–1183. [DOI] [PubMed] [Google Scholar]
- 13. Stone KL, et al. ; Osteoporotic Fractures in Men Study Group. Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J Am Geriatr Soc. 2014;62(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ensrud KE, et al. . Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13(10):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ensrud KE, et al. ; Study of Osteoporotic Fractures Research Group. Frailty and risk of falls, fracture, and mortality in older women: the Study of Osteoporotic Fractures. J Gerontol A Biol Sci Med Sci. 2007;62(7):744–751. [DOI] [PubMed] [Google Scholar]
- 16. Hall MH, et al. . Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the health, aging and body composition study. Sleep. 2015;38(2):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smagula SF, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group. Actigraphy- and polysomnography-measured sleep disturbances, inflammation, and mortality among older men. Psychosom Med. 2016;78(6):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stone KL, et al. . Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57(4):604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foley D, et al. . Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. [DOI] [PubMed] [Google Scholar]
- 20. Spira AP, et al. . Association between insomnia symptoms and functional status in U.S. older adults. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 1):S35–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stenholm S, et al. . Self-reported sleep duration and time in bed as predictors of physical function decline: results from the InCHIANTI study. Sleep. 2011;34(11):1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diaz K, et al. . Obstructive sleep apnea is associated with higher healthcare utilization in elderly patients. Ann Thorac Med. 2014;9(2):92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kao LT, et al. . Healthcare service utilization by patients with obstructive sleep apnea: a population-based study. PLoS One. 2015;10(9):e0137459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapur VK, et al. ; Sleep Heart Health Research Group. The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25(3):289–296. [PubMed] [Google Scholar]
- 25. Kaufmann CN, et al. . Insomnia and health services utilization in middle-aged and older adults: results from the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2013;68(12):1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novak M, et al. . Increased utilization of health services by insomniacs—an epidemiological perspective. J Psychosom Res. 2004;56(5):527–536. [DOI] [PubMed] [Google Scholar]
- 27. Ozminkowski RJ, et al. . The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273. [DOI] [PubMed] [Google Scholar]
- 28. Tarasiuk A, et al. . The effect of obstructive sleep apnea on morbidity and health care utilization of middle-aged and older adults. J Am Geriatr Soc. 2008;56(2):247–254. [DOI] [PubMed] [Google Scholar]
- 29. Buysse DJ, et al. . Can an improvement in sleep positively impact on health? Sleep Med Rev. 2010;14(6):405–410. [DOI] [PubMed] [Google Scholar]
- 30. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cummings SR, et al. . Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263(5):665–668. [PubMed] [Google Scholar]
- 32. Schousboe JT, et al. . Magnitude and consequences of misclassification of incident hip fractures in large cohort studies: the Study of Osteoporotic Fractures and Medicare claims data. Osteoporos Int. 2013;24(3):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schousboe JT, et al. . Estimation of standardized hospital costs from Medicare claims that reflect resource requirements for care: impact for cohort studies linked to Medicare claims. Health Serv Res. 2014;49(3):929–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 35. Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9(1):5–11. [DOI] [PubMed] [Google Scholar]
- 36. Schousboe JT, et al. . Pre-fracture individual characteristics associated with high total health care costs after hip fracture. Osteoporos Int. 2017;28(3):889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schousboe JT, et al. . Estimating true resource costs of outpatient care for Medicare beneficiaries: standardized costs versus Medicare payments and charges. Health Serv Res. 2016;51(1):205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheikh J, et al. . Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986:165–173. [Google Scholar]
- 39. Crum RM, et al. . Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 40. Pahor M, et al. . Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. [DOI] [PubMed] [Google Scholar]
- 41. Manning WG, et al. . Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. [DOI] [PubMed] [Google Scholar]
- 42. Pregibon D. Goodness of link tests for generalized linear models. Appl Stat. 1980;29(1):15–24. [Google Scholar]
- 43. Paudel ML, et al. . Sleep disturbances and risk of hospitalization and inpatient days among older women. Sleep. 2017;40(2). doi:10.1093/sleep/zsx037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Furihata R, et al. . An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3). doi:10.1093/sleep/zsw075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Newhouse JP, et al. . Steps to reduce favorable risk selection in Medicare advantage largely succeeded, boding well for health insurance exchanges. Health Aff (Millwood). 2012;31(12):2618–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.