Figure 8. PI4,5P2 binding to p53 controls small heat shock protein binding to p53 in vitro.
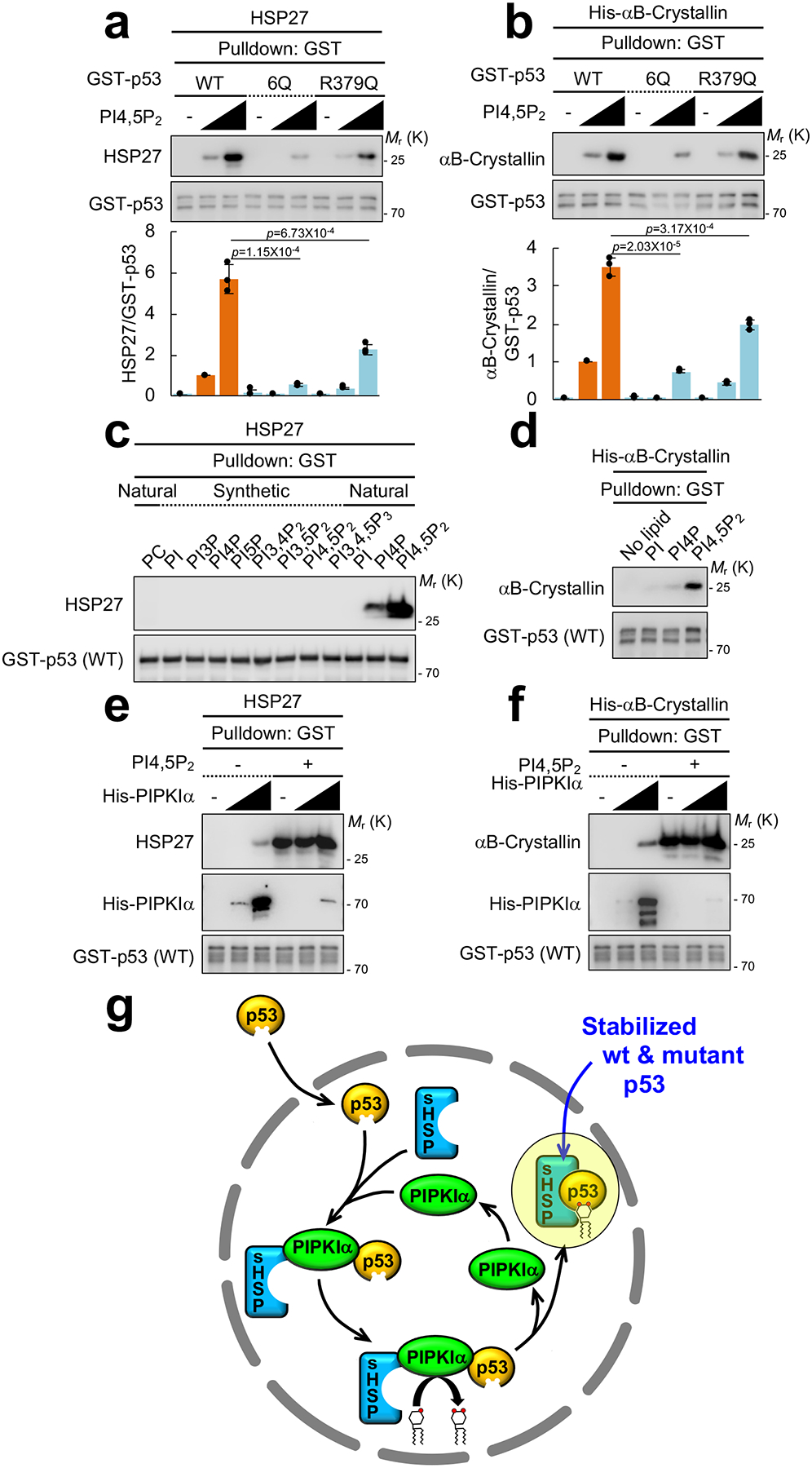
(A and B) Recombinant 0.1 μM GST-p53 and 0.5 μM untagged HSP27 or His-αB-Crystallin were incubated with 0, 1, or 10 μM PI4,5P2. GST-p53 proteins were pulled down and the associated HSP27 or His-αB-Crystallin was analyzed with an anti-HSP27 or an anti-αB-Crystallin antibody. The graph is shown as mean ± SD of n=3 independent experiments. Two-sided paired Student t-tests were used for statistical analysis (*, p<0.05; **, p<0.01).
(C) 0.1 μM GST-p53 and 0.5 μM untagged HSP27 were incubated with 2.0 μM liposomes containing the indicated phosphoinositides. GST-p53 was pulled down and the associated HSP27 was analyzed by IB. Representative data of n=3 independent experiments were shown.
(D) 0.1 μM GST-p53 and 0.5 μM His-αB-Crystallin were incubated with 1 μM PI, PI4P, or PI4,5P2. GST-p53 was pulled down and the associated His-αB-Crystallin was analyzed by IB. Representative data of n=3 independent experiments were shown.
(E and F) 0.1 μM GST-p53, 0.5 μM untagged HSP27 or His-αB-Crystallin and 0, 0.01 and 0.1 μM His-PIPKIα were incubated with 1 μM PI4,5P2. GST-p53 was pulled down and the associated HSP27 or His-αB-Crystallin and His-PIPKIα was analyzed by IB. Representative data of n=3 independent experiments were shown.
(G) A schematic model of how p53 stability is regulated by PIPKIα, PI4,5P2 and small heat shock proteins (sHSPs).
