Abstract
Objective
Hepatocellular carcinoma (HCC) has become a pressing health problem facing the world today due to its high morbidity, high mortality, and late discovery. As a diagnostic criteria of HCC, the exact threshold of Alpha-fetoprotein (AFP) is controversial. Therefore, this study was aimed to systematically estimate the performance of AFP in diagnosing HCC and to clarify its optimal threshold.
Methods
Medline and Embase databases were searched for articles indexed up to November 2019. English language studies were included if both the sensitivity and specificity of AFP in the diagnosis of HCC were provided. The basic information and accuracy data included in the studies were extracted. Combined estimates for sensitivity and specificity were statistically analyzed by random-effects model using MetaDisc 1.4 and Stata 15.0 software at the prespecified threshold of 400 ng/mL, 200 ng/mL, and the range of 20–100 ng/mL. The optimal threshold was evaluated by the area under curve (AUC) of the summary receiver operating characteristic (SROC).
Results
We retrieved 29,828 articles and included 59 studies and 1 review with a total of 11,731 HCC cases confirmed by histomorphology and 21,972 control cases without HCC. The included studies showed an overall judgment of at risk of bias. Four studies with AFP threshold of 400 ng/mL showed the summary sensitivity and specificity of 0.32 (95%CI 0.31–0.34) and 0.99 (95%CI 0.98–0.99), respectively. Four studies with AFP threshold of 200 ng/mL showed the summary sensitivity and specificity of 0.49 (95%CI 0.47–0.50) and 0.98 (95%CI 0.97–0.99), respectively. Forty-six studies with AFP threshold of 20–100 ng/mL showed the summary sensitivity and specificity of 0.61 (95%CI 0.60–0.62) and 0.86 (95%CI 0.86–0.87), respectively. The AUC of SROC and Q index of 400 ng/mL threshold were 0.9368 and 0.8734, respectively, which were significantly higher than those in 200 ng/mL threshold (0.9311 and 0.8664, respectively) and higher than those in 20–100 ng/mL threshold (0.8330 and 0.7654, respectively). Furthermore, similar result that favored 400 ng/mL were shown in the threshold in terms of AFP combined with ultrasound.
Conclusion
AFP levels in serum showed good accuracy in HCC diagnosis, and the threshold of AFP with 400 ng/mL was better than that of 200 ng/mL in terms of sensitivity and specificity no matter AFP is used alone or combined with ultrasound.
Introduction
Hepatocellular carcinoma (HCC) remains one of the most invasive cancers in humans, mostly occurring in patients with chronic liver disease, and the third leading cause of cancer-related death throughout the world [1]. Although its causes, prevention, and treatment strategies are recommended in guidelines, HCC is expected to become a pressing health problem facing the world in the coming decades [1, 2] Although researchers are making strides in HCC monitoring and treatment, there has been little improvement in survival in patients with HCC. In the United States, the 5-year survival rate of patients with HCC is still less than 12% [3]. The effective therapies are very limited for advanced HCC whose the survival rate decreased significantly [4], while there are several available treatments for the management of HCC with early stage, such as radical resection or liver transplantation, where 5-year survival rate of HCC patients who met the Milan criteria (single nodule < 5cm or three nodules diameter < 3cm) after liver transplantation was more than 70% [5, 6]. Therefore, the early discovery of HCC might be very important, and it is reported that early detection of HCC can improve the clinical outcomes [7]. Based on the evidence of benefits from early detection of HCC, the guidelines of both American Association, Asian Pacific Association, and Japan Association recommend HCC monitoring in high-risk patients for early diagnosis of HCC [8–11].
The alpha-fetoprotein (AFP) in serum is currently available diagnostic marker for HCC discovery. As for patients with chronic liver disease, a sustained increase in AFP serum level was shown to be one of the risk factors of HCC and has been used to help identify high-risk subgroup of chronic liver disease [12]. In patients with liver cirrhosis, fluctuations in AFP levels may reflect the sudden onset of viral hepatitis, the deterioration of the potential liver disease, or the development of HCC [13]. Besides, the level of AFP was reported to interact with some molecular subtypes such as EpCAM positive in invasive HCC [14–16]. It is established that multiple factors could contribute to the AFP level, which increases the difficulty of identifying the threshold. When the cutoff value of AFP was 20 ng/ml, the detection showed relatively good sensitivity with poor specificity, while when the cutoff value was 200 ng/ml, the discovery performed high specificity, but the sensitivity decreased significantly [17]. In 2001 and 2017 diagnostic staging standard of HCC in China, AFP 400 ng/mL was used as the diagnostic threshold [18]. However, a meta-analysis [19] shows that the diagnostic efficiency of AFP ≥ 200 ng/mL may be higher, partly because some of the early HCC [20] may be missed in the population with low concentration of AFP (20 to 200 ng/mL) if 400 ng/mL is still used as the criteria in HCC screening. Therefore, up to now, the optimal threshold of AFP for the diagnosis of HCC is still controversial [21–23].
In addition, it has been reported that AFP combined with ultrasound detection might improve the detection rate of HCC [24]. Both American Association for the Study of Liver Disease (AASLD) and European Association for the Study of the Liver (EASL) suggest that it is necessary to monitor HCC in high-risk patients partly by abdominal ultrasonography every six months, but there exists argument in the use of AFP as an auxiliary monitoring test and there is no identified threshold of AFP when the combination of AFP and ultrasound is used to monitor HCC [25, 26].
Therefore, it is particularly important to explore the optimal screening and diagnostic threshold of serum AFP with or without ultrasound for early diagnosis of HCC. The purpose of this study was to identify the optimal diagnostic threshold of serum AFP by systematic review and meta analysis. This article was performed based on Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [27, 28], and Qualitu assessment for studies of diagnostic accuracy (QUADAS-2) was used to evaluate the quality of diagnostic test [29].
Results
We retrieved 29,828 records from databases search, and assessed 21,464 records after deleting the duplication, and finally 59 original articles in terms of AFP alone and one systematic review in terms of AFP in combination with ultrasound [30–87] were enrolled for data synthesis, as is shown in Fig 1. This systematic review finally yielded information on a total of 11,731 HCC cases confirmed by histomorphology and 21,972 control cases without HCC.
Fig 1. Flow diagram of study selection.
Basic information and quality assessment
The basic information of the included studies was shown in Table 1. In all, we summarized the results from 4 studies using a AFP threshold of 400 ng/mL, and from 4 studies using a AFP threshold of 200 ng/mL, and 46 studies using a AFP threshold of 20–100 ng/mL. As for the sample, the serum was used to detect the AFP by forty-three studies, while the remaining used plasma. The included 59 researches were conducted in diverse countries, including China (n = 15), USA (n = 11), Japan (n = 9), Korea (n = 8), Egypt (n = 5), Italy (n = 2), Thailand (n = 2), France (n = 2), South Africa (n = 1), Turkey (n = 1), India (n = 1), Germany (n = 1), Indonesia (n = 1), and Australia (n = 1). Thirty-seven studies used samples from Asian while twenty-three studies used samples from Caucasian. As for the etiology of HCC, 16 studies [49, 51, 52, 55, 57, 59, 62, 64, 66, 69, 73, 75, 79–81, 86] only covered HBV or HCV hepatitis, one study was not available, and the remaining 42 studies [30–48, 50, 53, 54, 56, 58, 60, 61, 63, 65, 68, 70–72, 74, 76–78, 82–84, 86, 87] were mix which included HBV infection, HCV infection, alcohol and others. Shown were the estimates of sensitivity, specificity, true positive, false positive, false negative, true negative in terms of AFP in HCC diagnosis in the Table 2. The quality assessment by QUADAS-2 tool revealed a overall judgment of at low risk of bias for the included studies, which was shown in S3 Table. Specifically, domain of patient selection, index test, and flow and timing showed a low risk of bias, domain of reference standard showed a conclusion of potential for bias exits, and the applicability concerns were rated as low.
Table 1. Characteristics of studies included in the meta-analysis.
| Study | Year | Country | HCC/controls | Etiology | Assay type | Cut-off (ng/mL) | Sample type |
|---|---|---|---|---|---|---|---|
| King et al. [30] | 1989 | South Africa | 98/120 | MIX | ELISA | 20 | Serum |
| Takikawa et al. [31] | 1992 | Japan | 116/512 | MIX | ELISA | 20 | Plasma |
| Fujiyama et al. [32] | 1992 | Japan | 200/197 | MIX | ELISA | 20 | Plasma |
| Suehiro et al. [33] | 1994 | Japan | 185/118 | MIX | ELISA | 20 | Plasma |
| Grazi et al. [34] | 1995 | Italy | 111/116 | MIX | ELISA | 20 | Serum |
| Nomura et al. [35] | 1999 | Japan | 36/49 | MIX | ELISA | 20 | Serum |
| Sassa et al. [36] | 1999 | Japan | 61/134 | MIX | ELISA | 20 | Serum |
| Ishii et al. [37] | 2000 | Japan | 29/705 | MIX | ELISA | 20 | Serum |
| Cui et al. [38] | 2002 | China | 60/30 | MIX | ELISA | 20 | Serum |
| Shimizu et al. [39] | 2002 | Japan | 56/34 | MIX | ELISA | 20 | Serum |
| Cui et al. [40] | 2003 | China | 120/90 | MIX | ELISA | 20 | Serum |
| Marrero et al. [41] | 2003 | USA | 55/104 | MIX | ELISA | 20 | Serum |
| Marrero et al. [42] | 2005 | USA | 144/108 | MIX | ELISA | 99 | Serum |
| Wang et al. [43] | 2005 | China | 61/64 | MIX | ELISA | 20 | Serum |
| Kim et al. [44] | 2006 | Korea | 62/60 | MIX | CH | 70.4 | Plasma |
| Volk et al. [45] | 2007 | USA | 84/169 | MIX | ELISA | 23 | Serum |
| Durazo et al. [46] | 2008 | USA | 144/96 | MIX | ELISA | 25 | Serum |
| Beneduce et al. [47] | 2008 | Italy | 33/31 | MIX | ELISA | 20 | Serum |
| Wang et al. [48] | 2009 | USA | 164/113 | MIX | ELISA | NK | Serum |
| Hu et al. [49] | 2009 | China | 31/93 | HBV | ELISA | 36 | Serum |
| Marrero et al. [50] | 2009 | USA | 419/417 | MIX | ELISA | 20 | Serum |
| Yoon et al. [51] | 2009 | Korea | 106/100 | HBV | ELISA | 20 | Serum |
| Sterling et al. [52] | 2009 | USA | 74/298 | HCV | ELISA | 20 | Serum |
| Baek et al. [53] | 2009 | Korea | 227/100 | MIX | ELISA | 20 | Serum |
| Yamamoto et al. [54] | 2009 | Japan | 190/490 | MIX | ELISA | 20 | Serum |
| Mao et al. [55] | 2010 | China, USA | 789/3428 | HBV | ELISA | 35 | Serum |
| Ozkan et al. [56] | 2010 | Turkey | 75/83 | MIX | ELISA | 4.36 | Serum |
| Bessa et al. [57] | 2010 | Egypt | 30/30 | HCV | ELISA | 69.5 | Plasma |
| Sharma et al. [58] | 2010 | India | 70/38 | MIX | ELISA | 13 | Serum |
| Ishida et al. [59] | 2010 | Japan | 141/143 | HCV | ELISA | 20 | Serum |
| Tian et al. [60] | 2011 | China | 153/219 | MIX | ELISA | 13.6 | Serum |
| Shi et al. [61] | 2011 | China | 55/107 | MIX | ELISA | 400 | Serum |
| Makarem et al. [62] | 2011 | Egypt | 113/120 | HCV | CH | 43 | Plasma |
| Morota et al. [63] | 2011 | USA | 70/34 | MIX | ELISA | 15 | Serum |
| Salem et al. [64] | 2012 | Egypt | 30/40 | HCV | ELISA | 10.4 | Serum |
| Shang-1 et al. [65] | 2012 | Thailand | 91/23 | MIX | ELISA | 20 | Plasma |
| Shang-2 et al. [65] | 2012 | USA | 40/73 | MIX | ELISA | 20 | Plasma |
| Yang et al. [66] | 2013 | China | 179/80 | HBV | CH | 20 | Plasma |
| Choi et al. [67] | 2013 | Korea | 90/78 | NA | ELISA | 10 | Serum |
| Ertle et al. [68] | 2013 | Germany | 164/422 | MIX | ELISA | 10 | Serum |
| Xu-1 et al. [69] | 2014 | China | 2472/578 | HBV | ELISA | 20 | Serum |
| Xu-2 et al. [69] | 2014 | China | 2472/578 | HBV | ELISA | 200 | Serum |
| Xu-3 et al. [69] | 2014 | China | 2472/578 | HBV | ELISA | 400 | Serum |
| Chan-1 et al. [70] | 2014 | China | 562/243 | MIX | CH | 10 | Serum |
| Chan-2 et al. [70] | 2014 | China | 562/243 | MIX | CH | 200 | Serum |
| Chan-3 et al. [70] | 2014 | China | 562/243 | MIX | CH | 500 | Serum |
| Gopal-1 et al. [71] | 2014 | USA | 452/676 | MIX | ELISA | 20 | Serum |
| Gopal-2 et al. [71] | 2014 | USA | 452/676 | MIX | ELISA | 200 | Serum |
| Gopal-3 et al. [71] | 2014 | USA | 452/676 | MIX | ELISA | 400 | Serum |
| Lee et al. [72] | 2014 | Korea | 120/40 | MIX | ELISA | 6 | Serum |
| Nabih et al. [73] | 2014 | Egypt | 35/34 | HCV | CH | 240 | Plasma |
| Song et al. [74] | 2014 | China | 550/604 | MIX | ELISA | 21 | Serum |
| Costa et al. [75] | 2015 | France | 75/75 | HCV | ELISA | 20 | Plasma |
| Poté et al. [76] | 2015 | France | 85/43 | MIX | ELISA | 5 | Serum |
| Chang et al. [77] | 2015 | China | 363/1234 | MIX | ELISA | 20 | Serum |
| Gani et al. [78] | 2015 | Indonesia | 59/47 | MIX | ELISA | 20.45 | Serum |
| Chimparlee et al. [79] | 2015 | Thailand | 157/170 | HBV | ELISA | 20 | Serum |
| Fouad et al. [80] | 2015 | Egypt | 25/25 | HCV | ELISA | 142 | Serum |
| Ge et al. [81] | 2015 | China | 89/301 | HBV | ELISA | 6.79 | Serum |
| Yu et al. [82] | 2015 | China | 134/347 | MIX | CLEIA | 20 | Serum |
| Jang et al. [83] | 2016 | Korea | 208/193 | MIX | ELISA | 20 | Plasma |
| Roslyn et al. [84] | 2016 | Australia | 86/258 | MIX | CH | 20 | Serum |
| Ji et al. cohort A [85] | 2016 | China | 236/135 | HBV | ELISA | 20 | Serum |
| Ji et al. cohort B [85] | 2016 | China | 200/97 | HBV | ELISA | 20 | Serum |
| Ahn-1 et al. [86] | 2016 | Korea | 366/366 | MIX | ELISA | 20 | Serum |
| Ahn-2 et al. [86] | 2016 | Korea | 366/366 | MIX | ELISA | 100 | Serum |
| Ahn-3 et al. [86] | 2016 | Korea | 366/366 | MIX | ELISA | 200 | Serum |
| Ahn-4 et al. [86] | 2016 | Korea | 366/366 | MIX | ELISA | 400 | Serum |
| Lim et al. [87] | 2016 | Korea | 361/276 | MIX | ELISA | 20 | Serum |
MIX: the etiology including HBV infection, HCV infection, alcohol and others; ELISA: enzyme immunometric assay; CH: chemiluminescence; CLEIA: chemiluminescence enzyme immunoassay; NK = not known; NA: not available.
Table 2. The indicators for HCC diagnosis were extracted from the included studies.
| Study | Year | SE (%) | SP (%) | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|
| King et al. [30] | 1989 | 74 | 99 | 73 | 1 | 25 | 119 |
| Takikawa et al. [31] | 1992 | 71 | 75 | 82 | 128 | 34 | 384 |
| Fujiyama et al. [32] | 1992 | 51 | 97 | 102 | 6 | 98 | 191 |
| Suehiro et al. [33] | 1994 | 65 | 72 | 120 | 33 | 65 | 85 |
| Grazi et al. [34] | 1995 | 55 | 97 | 61 | 3 | 50 | 113 |
| Nomura et al. [35] | 1999 | 58 | 76 | 21 | 12 | 15 | 37 |
| Sassa et al. [36] | 1999 | 8 | 100 | 5 | 0 | 56 | 134 |
| Ishii et al. [37] | 2000 | 62 | 78 | 18 | 155 | 11 | 550 |
| Cui et al. [38] | 2002 | 59 | 85 | 35 | 4 | 25 | 26 |
| Shimizu et al. [39] | 2002 | 57 | 63 | 32 | 13 | 24 | 21 |
| Cui et al. [40] | 2003 | 93 | 63 | 112 | 33 | 8 | 57 |
| Marrero et al. [41] | 2003 | 67 | 86 | 37 | 15 | 18 | 89 |
| Marrero et al. [42] | 2005 | 30 | 96 | 43 | 4 | 101 | 104 |
| Wang et al. [43] | 2005 | 59 | 77 | 36 | 15 | 25 | 49 |
| Kim et al. [44] | 2006 | 54.8 | 100 | 34 | 0 | 28 | 60 |
| Volk et al. [45] | 2007 | 62 | 91 | 52 | 15 | 32 | 154 |
| Durazo et al. [46] | 2008 | 48 | 87 | 69 | 12 | 75 | 84 |
| Beneduce et al. [47] | 2008 | 69 | 88 | 23 | 4 | 10 | 27 |
| Wang et al. [48] | 2009 | 95 | 21 | 156 | 89 | 8 | 24 |
| Hu et al. [49] | 2009 | 48 | 97 | 15 | 3 | 16 | 90 |
| Marrero et al. [50] | 2009 | 59 | 90 | 247 | 42 | 172 | 375 |
| Yoon et al. [51] | 2009 | 61 | 71 | 65 | 29 | 41 | 71 |
| Sterling et al. [52] | 2009 | 55 | 77 | 41 | 69 | 33 | 229 |
| Baek et al. [53] | 2009 | 51 | 91 | 116 | 9 | 111 | 91 |
| Yamamoto et al. [54] | 2009 | 58 | 88 | 110 | 59 | 80 | 431 |
| Mao et al. [55] | 2010 | 58 | 85 | 458 | 514 | 331 | 2914 |
| Ozkan et al. [56] | 2010 | 83 | 95 | 62 | 4 | 13 | 79 |
| Bessa et al. [57] | 2010 | 60 | 90 | 18 | 3 | 12 | 27 |
| Sharma et al. [58] | 2010 | 73 | 66 | 51 | 13 | 19 | 25 |
| Ishida et al. [59] | 2010 | 52 | 61 | 73 | 56 | 68 | 87 |
| Tian et al. [60] | 2011 | 95 | 47 | 145 | 116 | 8 | 103 |
| Shi et al. [61] | 2011 | 38 | 93 | 21 | 7 | 34 | 100 |
| Makarem et al. [62] | 2011 | 74 | 100 | 84 | 0 | 29 | 120 |
| Morota et al. [63] | 2011 | 63 | 91 | 44 | 3 | 26 | 31 |
| Salem et al. [64] | 2012 | 90 | 78 | 27 | 9 | 3 | 31 |
| Shang-1 et al. [65] | 2012 | 53 | 93 | 21 | 5 | 19 | 68 |
| Shang-2 et al. [65] | 2012 | 78 | 96 | 71 | 1 | 20 | 22 |
| Yang et al. [66] | 2013 | 37 | 85 | 66 | 12 | 113 | 68 |
| Choi et al. [67] | 2013 | 79 | 85 | 71 | 12 | 19 | 66 |
| Ertle et al. [68] | 2013 | 55 | 95 | 90 | 21 | 74 | 401 |
| Xu-1 et al. [69] | 2014 | 69.74 | 91.18 | 1724 | 51 | 748 | 527 |
| Xu-2 et al. [69] | 2014 | 51.58 | 97.75 | 1275 | 13 | 1197 | 565 |
| Xu-3 et al. [69] | 2014 | 31.47 | 99.13 | 778 | 5 | 1694 | 573 |
| Chan-1 et al. [70] | 2014 | 82.6 | 70.4 | 464 | 72 | 98 | 171 |
| Chan-2 et al. [70] | 2014 | 47.7 | 97.1 | 268 | 7 | 294 | 236 |
| Chan-3 et al. [70] | 2014 | 38.1 | 100 | 214 | 0 | 348 | 243 |
| Gopal-1 et al. [71] | 2014 | 70.1 | 89.8 | 317 | 69 | 135 | 607 |
| Gopal-2 et al. [71] | 2014 | 50 | 99.4 | 226 | 4 | 226 | 672 |
| Gopal-3 et al. [71] | 2014 | 44 | 99.9 | 199 | 1 | 253 | 675 |
| Lee et al. [72] | 2014 | 64 | 95 | 77 | 2 | 43 | 38 |
| Nabih et al. [73] | 2014 | 49 | 91 | 17 | 3 | 18 | 31 |
| Song et al. [74] | 2014 | 61 | 93 | 336 | 42 | 214 | 562 |
| Costa et al. [75] | 2015 | 49 | 87 | 37 | 11 | 38 | 65 |
| Poté et al. [76] | 2015 | 68 | 51 | 58 | 21 | 27 | 22 |
| Chang et al. [77] | 2015 | 53 | 93 | 192 | 83 | 171 | 1151 |
| Gani et al. [78] | 2015 | 73 | 92 | 43 | 4 | 16 | 43 |
| Chimparlee et al. [79] | 2015 | 67 | 97 | 105 | 5 | 52 | 165 |
| Fouad et al. [80] | 2015 | 100 | 100 | 25 | 0 | 0 | 25 |
| Ge et al. [81] | 2015 | 72 | 88 | 64 | 36 | 25 | 265 |
| Yu et al. [82] | 2015 | 77 | 65 | 103 | 121 | 31 | 226 |
| Jang et al. [83] | 2016 | 62 | 90 | 129 | 19 | 79 | 174 |
| Roslyn et al. [84] | 2016 | 43 | 97 | 37 | 9 | 49 | 249 |
| Ji et al. cohort A [85] | 2016 | 68 | 81 | 160 | 26 | 76 | 109 |
| Ji et al. cohort B [85] | 2016 | 62 | 69 | 124 | 30 | 76 | 67 |
| Ahn-1 et al. [86] | 2016 | 50.55 | 87.7 | 185 | 45 | 181 | 321 |
| Ahn-2 et al. [86] | 2016 | 37.7 | 95.9 | 138 | 15 | 228 | 351 |
| Ahn-3 et al. [86] | 2016 | 30.05 | 97.27 | 110 | 10 | 256 | 356 |
| Ahn-4 et al. [86] | 2016 | 24.04 | 98.36 | 88 | 6 | 278 | 360 |
| Lim et al. [87] | 2016 | 56.8 | 82.8 | 205 | 47 | 156 | 229 |
SE: sensitivity; Sp: specificity; TP: true positive; FP: false positive; FN: false negative; TN: true negative.
Meta-analysis of diagnostic accuracy estimates
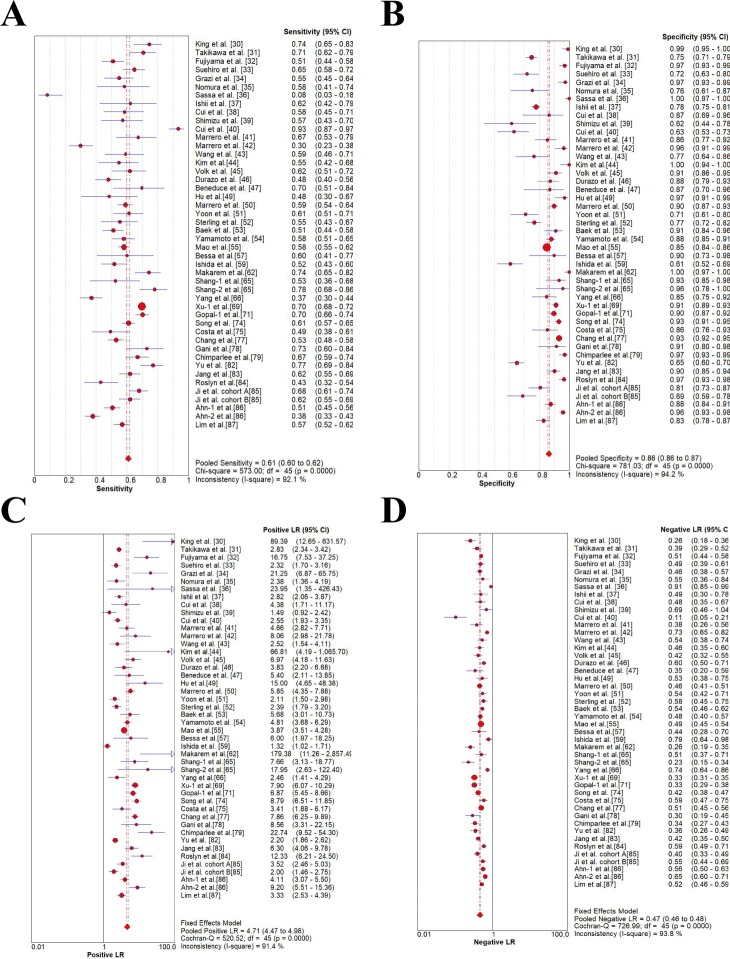
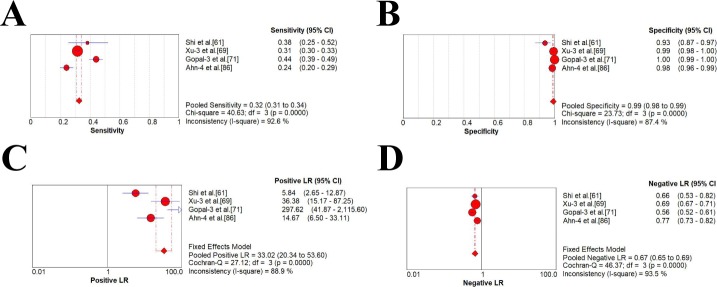
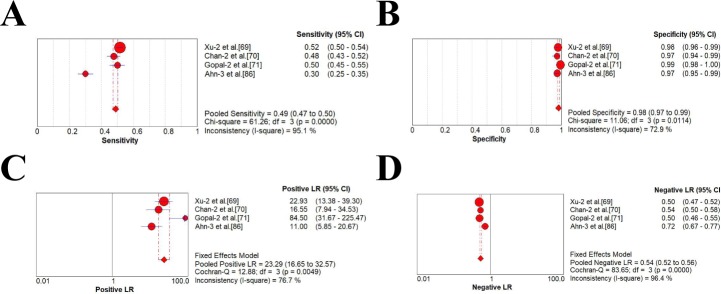
As was shown in Table 3 and Figs 2–4, four studies with AFP threshold of 400 ng/mL showed the summary sensitivity and specificity of 0.32 (95%CI 0.31–0.34) and 0.99 (95%CI 0.98–0.99), respectively, while eighteen studies with 400 ng/mL plus ultrasound showed the pooled sensitivity and specificity of 0.41 (95%CI 0.39–0.43) and 0.94 (95%CI 0.93–0.94), respectively. Four studies with AFP threshold of 200 ng/mL showed the summary sensitivity and specificity of 0.49 (95%CI 0.47–0.50) and 0.98 (95%CI 0.97–0.99), respectively, while eighteen studies with 200 ng/mL plus ultrasound showed the pooled sensitivity and specificity of 0.54 (0.52–0.55) and 0.94 (0.93–0.94), respectively. Forty-six studies with AFP threshold of 20–100 ng/mL showed the summary sensitivity and specificity of 0.61 (95%CI 0.60–0.62) and 0.86 (95%CI 0.86–0.87), respectively, while sixty studies eighteen studies with 20–100 ng/mL plus ultrasound showed the pooled sensitivity and specificity of 0.62 (0.61–0.63) and 0.88 (0.88–0.89), respectively.
Table 3. Diagnostic accuracy estimates based on varied thresholds of AFP.
| Cut-off Value (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| 20–100 | 200 | 400 | ||||
| AFP | AFP+US | AFP | AFP+US | AFP | AFP+US | |
| Sensitivity | 0.61 (0.60–0.62) | 0.62 (0.61–0.63) | 0.49 (0.47–0.50) | 0.54 (0.52–0.55) | 0.32 (0.31–0.34) | 0.41 (0.39–0.43) |
| Specificity | 0.86 (0.86–0.87) | 0.88 (0.88–0.89) | 0.98 (0.97–0.99) | 0.94 (0.93–0.94) | 0.99 (0.98–0.99) | 0.94 (0.93–0.94) |
| +LR | 4.71 (4.47–4.98) | 5.13 (4.89–5.38) | 23.29 (16.65–32.57) | 13.63 (11.86–15.67) | 33.02 (20.34–53.6) | 13.28 (11.59–15.23) |
| -LR | 0.47 (0.46–0.48) | 0.44 (0.43–0.45) | 0.54 (0.52–0.56) | 0.43 (0.41–0.45) | 0.67 (0.65–0.69) | 0.50 (0.48–0.52) |
| dOR | 10.64 (9.91–11.42) | 12.25 (11.46–13.10) | 42.06 (29.88–59.20) | 46.65 (37.62–57.84) | 47.63 (29.22–77.64) | 50.56 (39.58–64.58) |
| AUC | 0.8330 | 0.8464 | 0.9311 | 0.9359 | 0.9368 | 0.9394 |
| SE(AUC) | 0.0036 | 0.0032 | 0.0084 | 0.0049 | 0.0111 | 0.0054 |
| Q* | 0.7654 | 0.7778 | 0.8664 | 0.8723 | 0.8734 | 0.8767 |
| SE(Q*) | 0.0033 | 0.0030 | 0.0101 | 0.0061 | 0.0138 | 0.0068 |
AFP alpha-fetoprotein, US ultrasound, +LR positive likelihood ratio, -LR negative likelihood ratio, dOR diagnostic odds ratio, AUC area under curve, SE standard error
Fig 2. Forest plots of the estimates for AFP in HCC diagnosis (20–100 ng/mL).
(A) Pooled sensitivity. (B) Pooled specificity. (C) Pooled positive LR. (D) Pooled negative LR.
Fig 4. Forest plots of the estimates for AFP in HCC diagnosis (400 ng/mL).
(A) Pooled sensitivity. (B) Pooled specificity. (C) Pooled positive LR. (D) Pooled negative LR.
Fig 3. Forest plots of the estimates for AFP in HCC diagnosis (200 ng/mL).
(A) Pooled sensitivity. (B) Pooled specificity. (C) Pooled positive LR. (D) Pooled negative LR.
The result from AFP alone as the marker indicated that the specificity of the threshold 400 ng/mL was the highest (99.0%), but the sensitivity was the lowest (32.0%). The specificity of the 200 ng/mL was 1.0% lower than that of the 400 ng/mL, but the sensitivity could increase to 49.0%, with dOR being the highest (42.06%). The threshold of 20–100 ng/mL owned the greatest sensitivity of 61.0%, but the specificity and dOR were lower than that of 200 ng/mL and 400 ng/mL.
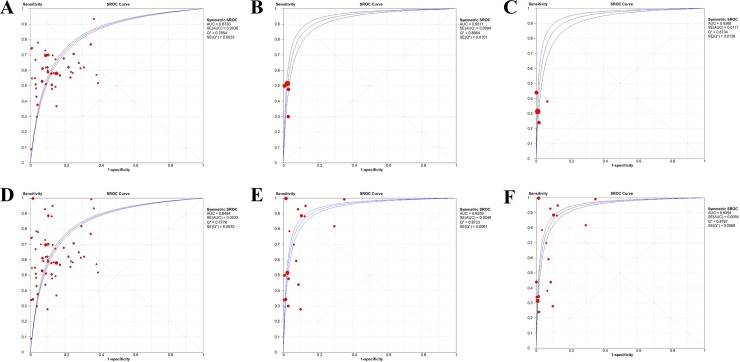
Threshold identification by SROC analysis
As is shown in Table 3 and Fig 5, The AUC of SROC and Q index of 400 ng/mL threshold were 0.9368 and 0.8734, respectively, which were significantly higher than those in 200 ng/mL threshold (0.9311 and 0.8664, respectively) and higher than those in 20-100ng/mL threshold (0.8330 and 0.7654, respectively). Similarly, when combined with ultrasound, the AUC of SROC and Q index of 400 ng/mL threshold were 0.9394 and 0.8767, respectively, which were significantly higher than those in 200 ng/mL threshold (0.9359 and 0.8723, respectively) and higher than those in 20–100 ng/mL threshold (0.8464 and 0.7778, respectively).
Fig 5. Summary receiver operating characteristic curves (SROC).
(A). SROC curve for AFP in 20–100 ng/mL. (B). SROC curve for AFP in 200 ng/mL. (C). SROC curve for AFP in 400 ng/mL. (D) SROC curve for AFP in 20–100 ng/mL combined with ultrasound. (E) SROC curve for AFP in 200 ng/mL combined with ultrasound. (F) SROC curve for AFP in 400 ng/mL combined with ultrasound.
Heterogeneity test and meta-regression analysis
There was no heterogeneity between groups of different threshold (p > 0.05), as was shown in Table 4. However, there existed heterogeneity in sensitivity, specificity, + LR, -LR and dOR within groups with varied threshold, as was shown in Table 5. This heterogeneity may be related to the diversity of population selection, including hepatitis B (HBV) and hepatitis C (HCV), as well as some mixed cases, along with diverse detection methods, instruments, reagents, standards. However, only indicators of potential heterogeneity sources such as control, year, country, sample type, assay type and etiology (HBV, HCV or MIX) could be extracted from the included articles. The P-value > 0.10 was realized as homogeneous [88], and no statistically significant effect existed on heterogeneity of three groups (P > 0.10), as shown in Table 6.
Table 4. Spearman correlation analysis results.
| Cut-off Value (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| 20–100 | 200 | 400 | ||||
| AFP | AFP+US | AFP | AFP+US | AFP | AFP+US | |
| Rs | 0.22 | 0.235 | -0.6 | 0.482 | -0.4 | 0.515 |
| p value | 0.142 | 0.071 | 0.4 | 0.043 | 0.6 | 0.029 |
AFP alpha-fetoprotein, US ultrasound, Rs rank correlation spearman
Table 5. Chi-square test and Cochrane-Q test results.
| Cut-off Value (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| 20–100 | 200 | 400 | ||||
| AFP | AFP+US | AFP | AFP+US | AFP | AFP+US | |
| Sensitivity | ||||||
| X2 | 573 | 1020.46 | 61.26 | 648.00 | 40.63 | 937.09 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Specificity | ||||||
| X2 | 781.03 | 1559.36 | 11.06 | 738.36 | 23.73 | 783.16 |
| p value | <0.0001 | <0.0001 | 0.0114 | <0.0001 | <0.0001 | <0.0001 |
| +LR | ||||||
| Cochrane-Q | 520.52 | 934.42 | 12.88 | 737.99 | 27.12 | 716.93 |
| p value | <0.0001 | <0.0001 | 0.0049 | <0.0001 | <0.0001 | <0.0001 |
| -LR | ||||||
| Cochrane-Q | 726.99 | 968.77 | 83.65 | 431.24 | 46.37 | 864.11 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| dOR | ||||||
| Cochrane-Q | 315.91 | 460.09 | 16.95 | 127.94 | 22.75 | 138.79 |
| p value | <0.0001 | <0.0001 | 0.0007 | <0.0001 | <0.0001 | <0.0001 |
+LR positive likelihood ratio, -LR negative likelihood ratio, dOR diagnostic odds ratio
Table 6. Meta-regression analyses of potential source of heterogeneity.
| Factors | Coeff. | Std. err. | P-value | RDOR |
|---|---|---|---|---|
| 20–100 ng/ml | ||||
| Year | 0.077 | 0.1606 | 0.6339 | 1.08 |
| Country | 0.062 | 0.0568 | 0.2819 | 1.06 |
| Control | 0.195 | 0.1531 | 0.2097 | 1.22 |
| Sample type | 0.030 | 0.2874 | 0.9169 | 1.03 |
| Etiology | 0.120 | 0.1410 | 0.3986 | 1.13 |
| Assay type | 0.022 | 0.2917 | 0.9409 | 1.02 |
| 200 ng/ml | ||||
| Country | -1.153 | 0.2972 | 0.1606 | 0.32 |
| Control | 1.195 | 0.9167 | 0.4165 | 3.3 |
| Etiology | -0.403 | 0.8508 | 0.7183 | 0.67 |
| 400 ng/ml | ||||
| Country | -0.495 | 0.5226 | 0.5173 | 0.61 |
| Control | 1.055 | 1.3062 | 0.5676 | 2.87 |
| Etiology | 0.104 | 0.7493 | 0.9119 | 1.11 |
Coeff coefficient, RDOR ratio of the diagnostic odds ratio.
Publication bias
Deek’s funnel plot showed a slope coefficient of 3.59 (p = 0.534), -42.60 (p = 0.666), -33.98 (p = 0.691) for included studies with 20–100, 200, 400 ng/mL, respectively, which indicated symmetry in data, where publication bias was not suggestive (S1–S3 Figs, online supplement).
Discussion
The disagreement between different international guidelines in terms of the AFP threshold for HCC diagnosis has been continued for several decades, and it has not yet been revolved so far. This article comprehensively reviewed the evidence for the threshold of AFP, and the results showed that AFP threshold of 400 ng/mL reporting the summary sensitivity of 0.32 (95%CI 0.31–0.34) and specificity of 0.99 (95%CI 0.98–0.99), was better than those of the threshold of 200 ng/mL (sensitivity of 0.49 (95%CI 0.47–0.50) and specificity of 0.98 (95%CI 0.97–0.99)), and better than those of the threshold of 20–100 ng/mL (sensitivity of 0.61 (95%CI 0.60–0.62) and specificity of 0.86 (95%CI 0.86–0.87)). The AUC of SROC and Q index of 400 ng/mL threshold were 0.9368 and 0.8734, respectively, which were significantly higher than those in 200 ng/mL threshold (0.9311 and 0.8664, respectively) and higher than those in 20–100 ng/mL threshold (0.8330 and 0.7654, respectively). Besides, similar result that favored 400 ng/mL were shown in the threshold in terms of AFP combined with ultrasound. The overall result indicated that the application of the AFP threshold of 400 ng/mL should be recommended for the diagnosis of HCC no matter it is used alone or combined with ultrasound to monitor the HCC.
It is well established that AFP level has been an optimal diagnostic marker for early diagnosis of HCC because of its well performance of sensitivity and specificity. However, along with HCC, there are other tumor contributors to the rise of AFP levels, such as reproductive system tumors; besides, the process of liver cell regeneration after an acute inflammation could also lead to the occurrence of a sharp increase in AFP levels during the progress of chronic liver diseases like hepatitis and liver cirrhosis[89–91]. Therefore, further laboratory examinations and imaging tests should be provided to combine the result of AFP to make a definite diagnosis [92, 93]. Because of this, the AFP threshold for the diagnosis of HCC is still controversial. AFP ≥ 400 ng/mL is recommended as the diagnostic criteria of HCC in the Chinese guideline for diagnosis and treatment of primary liver cancer (2017 edition) [94]. Nevertheless, Cedrone et al. [95] reported that the level of AFP in patients who had HCC was not affected by HBV or HCV, and a better threshold of serum AFP level should be 50 ng/mL. Another voice from Xu Jianye et al. [96] proposed that the 150 ng/mL diagnostic threshold of AFP for HCC showed better efficacy. Moreover, Zhang Jianhua et al. [20] proved that a low concentration of AFP in the range of 20–200 ng/mL could be used for early screening in the high risk population which could also be combined with ultrasound. However, the 2011 American Society of Hepatology HCC guidelines no longer use AFP as a screening method for HCC [97]. But what should draw our great attentions is the fact that unlike American, the major cause of HCC in other countries such as China is viral hepatitis, so that the dynamic surveillance of AFP level along with ultrasound in the screening among HCC high-risk population [98] still owns its great clinical application [99, 100]. What should actually be addressed in the next version guidelines of America, Europe, Asian-Pacific, and China, is the threshold of AFP in different phase in HCC management.
This meta analysis has its strengths and limitations. This systematic review included 59 articles and a total of 11,731 HCC cases and 21,972 non-HCC cases, which has summarized the evidence from the largest number of researches and participants representative of varied population from all over the world up to now. All the positive and negative cases in this review were confirmed by histomorphology, which ruled out the misclassification bias, and the quality of the included researches showed a low risk of bias. However, there is not without limitations. The articles in this meta analysis was restricted to the publications only in English language, which might missed the studies published in other languages. What is worth mentioning, in this review there are 20,732 cases from Asia, 630 cases from Africa, 5,924 cases from Europe, 8,666 cases from North America, which means that there might be selection bias when giving the conclusion of this article to the whole population; however, the results from meta-regression to detect the heterogeneity sources did not find any significant difference between countries. Furthermore, we have also detected considerable heterogeneity between three groups of varied threshold, and the meta-regression model has not discovered any heterogeneity resource with statistical significance. There also exists potential imbalance between the three groups of different threshold in terms of the number of the studies in each threshold group.
In conclusion, the present meta analysis suggests that AFP levels show good accuracy in HCC diagnosis, and the threshold of AFP with 400 ng/mL is better than that of 200 ng/mL and 20–100 ng/mL in terms of sensitivity and specificity no matter AFP is used alone or combined with ultrasound. Although included studies showed a low risk of bias, and publication bias was not suggestive, yet heterogeneity existed within groups, which might lead to the different threshold across geographic regions. Despite the current conclusion that AFP threshold of 400 ng/mL should be used for the diagnosis of HCC, the threshold of 20 ng/mL should also be suggested to lead to the decision to let a patient go into the surveillance program for HCC due to its high sensitivity. Future studies should pay more attention to the dynamic change of AFP along with the advance of HCC, where artificial intelligence might be applied to construct a model to predict the prognosis of HCC.
Materials and methods
This systematic review was performed according to the MOOSE and reported in accordance with PRISMA statement [27, 28]. The protocol was registered at PROSPERO (CRD42019133742, http://www.crd.york.ac.uk/PROSPERO).
Search strategy and article screening
The Medline and EMBASE databases were searched from inception up to November 2019 with the following terms: "alpha-Fetoproteins or AFP" AND "Carcinoma, Hepatocellular or Hepatocellular Carcinomas or Liver Cell Carcinoma" (The detailed search strategy was described in S1 Table and S2 Table). Besides, we reviewed the references in identified projects for further potential studies. Two reviewers independently screened the titles and abstracts of all retrieved records to find potentially appropriate studies, and then by reading the full text they evaluated the remaining records to identify studies suitable for data synthesis. Any disagreement was resolved by consensus or arbitrator.
Inclusion criteria
We finally included original articles that met the following criteria:
Type of the study was diagnostic accuracy study.
Participants in the study included both the patients with HCC diagnosed by pathological diagnosis (gold standard) were taken as the case group and the patients with clinically diagnosed non-liver cancer as the control group.
Indicators to be evaluated in the study included AFP.
There was a definite AFP measurement value in the article.
Complete diagnostic four-grid table data could be obtained from the literature. (the indicators for HCC diagnosis should be directly or indirectly calculated or extracted, including true negative (TN) value, false negative (FN) value, false positive (FP)value, the true positive (TP) value, specificity, and sensitivity)
Exclusion criteria
Non-English published studies.
Conference abstracts, reviews, comments, opinions, letters, and editorials.
Case reports, biochemical and experimental studies.
The sample detected in the study was not plasma or serum.
Information extraction and quality assessment
Basic information of each included studies was extracted by two reviewers independently. The QUADAS-2 was used to evaluate the quality of diagnostic test literature by two reviewers independently [29]. The evaluation tool includes three aspects—variation, bias, and report quality—and eleven items, where the answer of each item consists of three choices: "Yes," "No," and "unclear." "Yes" means the study meet the criterion, "No" means not satisfied or not mentioned, and "not clear" is partially satisfied or unable to obtain sufficient information from the literature.
Data extraction and statistical processing
The diagnostic four-grid table data including TN, FN, FP, and TP were extracted from the included literatures, and Meta Disc 1.4 as well as Stata 15.0 software were used for statistical processing. The random effect model was applied to summarize the accuracy estimates if there was heterogeneity, while the fixed-effect model was applied if there was not. We calculated summary estimates of sensitivity, specificity, diagnostic odds ratio (dOR), positive likelihood ratio (+ LR), negative likelihood ratio (- LR). A summary receiver operating characteristic (SROC) curve was also displayed and the area under curve (AUC), and Q * index was used to determine the threshold. Meta-analysis was used to obtain the combined value of the accuracy indicators and their 95%CI, The test level is α = 0.05. The heterogeneity caused by threshold effect was examined by Spearman correlation analysis, and sensitivity and specificity heterogeneity was examined by the chi-square test. The -LR and + LR were examined by Cochrane-Q test. Meta-regression analysis was used to detect the contributors of the heterogeneity. Deek’s funnel plot was used to assess the publication bias, and a slope coefficient with p <0.10 revealed significant bias.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by China National Science and Technology major projects 12th 5-year plan (No.2012ZX10005004), and Innovation team project of Beijing University of Chinese Medicine (2019-JYB-TD-009).
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76. Epub 2007/06/16. 10.1053/j.gastro.2007.04.061 . [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(9):1485–91. Epub 2009/02/20. 10.1200/jco.2008.20.7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. The New England journal of medicine. 2011;365(12):1118–27. Epub 2011/10/14. 10.1056/NEJMra1001683 . [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology (Baltimore, Md). 1999;29(1):62–7. Epub 1998/12/24. 10.1002/hep.510290145 . [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J. Early diagnosis and treatment of hepatocellular carcinoma. Bailliere's best practice & research Clinical gastroenterology. 2000;14(6):991–1008. Epub 2001/01/05. 10.1016/j.berh.2015.05.012 . [DOI] [PubMed] [Google Scholar]
- 6.Ioannou GN, Perkins JD, Carithers RL Jr., Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134(5):1342–51. Epub 2008/05/13. 10.1053/j.gastro.2008.02.013 . [DOI] [PubMed] [Google Scholar]
- 7.Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. The American journal of medicine. 2008;121(2):119–26. Epub 2008/02/12. 10.1016/j.amjmed.2007.09.020 . [DOI] [PubMed] [Google Scholar]
- 8.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. Epub 2012/03/20. 10.1016/j.jhep.2011.12.001 . [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md). 2018;68(2):723–50. Epub 2018/04/07. 10.1002/hep.29913 . [DOI] [PubMed] [Google Scholar]
- 10.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology international. 2010;4(2):439–74. Epub 2010/09/10. 10.1007/s12072-010-9165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Digestive diseases (Basel, Switzerland). 2011;29(3):339–64. Epub 2011/08/11. 10.1159/000327577 . [DOI] [PubMed] [Google Scholar]
- 12.Ciernik IF. Risk factors for hepatocellular carcinoma in patients with chronic liver diseases. The New England journal of medicine. 1993;329(25):1897; author reply -8. Epub 1993/12/16. . [PubMed] [Google Scholar]
- 13.Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43(3):434–41. Epub 2005/09/02. 10.1016/j.jhep.2005.03.019 . [DOI] [PubMed] [Google Scholar]
- 14.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer research. 2008;68(5):1451–61. Epub 2008/03/05. 10.1158/0008-5472.CAN-07-6013 . [DOI] [PubMed] [Google Scholar]
- 15.Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annual review of medicine. 2010;61:317–28. Epub 2010/01/12. 10.1146/annurev.med.080608.100623 ; PubMed Central PMCID: PMC3677155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer research. 2009;69(18):7385–92. Epub 2009/09/03. 10.1158/0008-5472.CAN-09-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34(4):570–5. Epub 2001/06/08. 10.1016/s0168-8278(00)00053-2 . [DOI] [PubMed] [Google Scholar]
- 18.Li Weidao, Revision of the criteria for diagnosis and staging of primary liver cancer. Chinese journal of general surgery, 2000(04): p. 46. (in Chinese) [Google Scholar]
- 19.Xu Jianye, Lin Ding, Li Weidao, et al. , Systematic evaluation of the accuracy of alpha-fetoprotein in diagnosis of primary liver cancer. Chinese journal of evidence-based medicine, 2009. 9(05): p. 525–530. (in Chinese) [Google Scholar]
- 20.Zhang Jianhuai, Ma Zengchen, Wang Jianying, Diagnosis and analysis of 424 cases of hepatocellular carcinoma with low concentration of alpha-fetoprotein. Chinese journal of general surgery, 2002(09): p. 36–37. (in Chinese) [Google Scholar]
- 21.Singal AG, Conjeevaram HS, Volk ML, Fu S, Fontana RJ, Askari F, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(5):793–9. Epub 2012/03/01. 10.1158/1055-9965.epi-11-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrero JA, El-Serag HB. Alpha-fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md). 2011;53(3):1060–1; author reply 1–2. Epub 2011/03/05. 10.1002/hep.24033 . [DOI] [PubMed] [Google Scholar]
- 23.Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Annals of internal medicine. 2012;156(5):387–9. Epub 2012/03/07. 10.7326/0003-4819-156-5-201203060-00012 . [DOI] [PubMed] [Google Scholar]
- 24.Tzartzeva K, Singal AG. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert review of gastroenterology & hepatology. 2018;12(10):947–9. Epub 2018/08/18. 10.1080/17474124.2018.1512855 . [DOI] [PubMed] [Google Scholar]
- 25.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md). 2018;67(1):358–80. Epub 2017/01/29. 10.1002/hep.29086 . [DOI] [PubMed] [Google Scholar]
- 26.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. Epub 2018/04/10. 10.1016/j.jhep.2018.03.019 . [DOI] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–12. Epub 2000/05/02. 10.1001/jama.283.15.2008 . [DOI] [PubMed] [Google Scholar]
- 28.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Annals of internal medicine. 2018;169(7):467–73. Epub 2018/09/05. 10.7326/M18-0850 . [DOI] [PubMed] [Google Scholar]
- 29.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529–36. Epub 2011/10/19. 10.7326/0003-4819-155-8-201110180-00009 . [DOI] [PubMed] [Google Scholar]
- 30.King MA, Kew MC, Kuyl JM, Atkinson PM. A comparison between des-gamma-carboxy prothrombin and alpha-fetoprotein as markers of hepatocellular carcinoma in southern African blacks. Journal of gastroenterology and hepatology. 1989;4(1):17–24. Epub 1989/01/01. 10.1111/j.1440-1746.1989.tb00802.x . [DOI] [PubMed] [Google Scholar]
- 31.Takikawa Y, Suzuki K, Yamazaki K, Goto T, Madarame T, Miura Y, et al. Plasma abnormal prothrombin (PIVKA-II): a new and reliable marker for the detection of hepatocellular carcinoma. Journal of gastroenterology and hepatology. 1992;7(1):1–6. Epub 1992/01/01. 10.1111/j.1440-1746.1992.tb00925.x . [DOI] [PubMed] [Google Scholar]
- 32.Fujiyama S, Izuno K, Yamasaki K, Sato T, Taketa K. Determination of optimum cutoff levels of plasma des-gamma-carboxy prothrombin and serum alpha-fetoprotein for the diagnosis of hepatocellular carcinoma using receiver operating characteristic curves. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 1992;13(5–6):316–23. Epub 1992/01/01. 10.1159/000217781 . [DOI] [PubMed] [Google Scholar]
- 33.Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, Maeda T. Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer. 1994;73(10):2464–71. Epub 1994/05/15. . [DOI] [PubMed] [Google Scholar]
- 34.Grazi GL, Mazziotti A, Legnani C, Jovine E, Miniero R, Gallucci A, et al. The role of tumor markers in the diagnosis of hepatocellular carcinoma, with special reference to the des-gamma-carboxy prothrombin. Liver transplantation and surgery: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 1995;1(4):249–55. Epub 1995/07/01. 10.1002/lt.500010410 . [DOI] [PubMed] [Google Scholar]
- 35.Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. The American journal of gastroenterology. 1999;94(3):650–4. Epub 1999/03/23. 10.1111/j.1572-0241.1999.00930.x . [DOI] [PubMed] [Google Scholar]
- 36.Sassa T, Kumada T, Nakano S, Uematsu T. Clinical utility of simultaneous measurement of serum high-sensitivity des-gamma-carboxy prothrombin and Lens culinaris agglutinin A-reactive alpha-fetoprotein in patients with small hepatocellular carcinoma. European journal of gastroenterology & hepatology. 1999;11(12):1387–92. Epub 2000/02/02. 10.1097/00042737-199912000-00008 . [DOI] [PubMed] [Google Scholar]
- 37.Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, et al. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. The American journal of gastroenterology. 2000;95(4):1036–40. Epub 2000/04/14. 10.1111/j.1572-0241.2000.01978.x . [DOI] [PubMed] [Google Scholar]
- 38.Cui R, Wang B, Ding H, Shen H, Li Y, Chen X. Usefulness of determining a protein induced by vitamin K absence in detection of hepatocellular carcinoma. Chinese medical journal. 2002;115(1):42–5. Epub 2002/04/05. . [PubMed] [Google Scholar]
- 39.Shimizu A, Shiraki K, Ito T, Sugimoto K, Sakai T, Ohmori S, et al. Sequential fluctuation pattern of serum des-gamma-carboxy prothrombin levels detected by high-sensitive electrochemiluminescence system as an early predictive marker for hepatocellular carcinoma in patients with cirrhosis. International journal of molecular medicine. 2002;9(3):245–50. Epub 2002/02/12. . [PubMed] [Google Scholar]
- 40.Cui R, He J, Zhang F, Wang B, Ding H, Shen H, et al. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. British journal of cancer. 2003;88(12):1878–82. Epub 2003/06/12. 10.1038/sj.bjc.6601018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology (Baltimore, Md). 2003;37(5):1114–21. Epub 2003/04/30. 10.1053/jhep.2003.50195 . [DOI] [PubMed] [Google Scholar]
- 42.Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43(6):1007–12. Epub 2005/09/03. 10.1016/j.jhep.2005.05.028 . [DOI] [PubMed] [Google Scholar]
- 43.Wang CS, Lin CL, Lee HC, Chen KY, Chiang MF, Chen HS, et al. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World journal of gastroenterology. 2005;11(39):6115–9. Epub 2005/11/08. 10.3748/wjg.v11.i39.6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. The American journal of gastroenterology. 2006;101(9):2051–9. Epub 2006/07/20. 10.1111/j.1572-0241.2006.00679.x . [DOI] [PubMed] [Google Scholar]
- 45.Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer biomarkers: section A of Disease markers. 2007;3(2):79–87. Epub 2007/05/25. 10.3233/cbm-2007-3202 . [DOI] [PubMed] [Google Scholar]
- 46.Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2008;23(10):1541–8. Epub 2008/04/22. 10.1111/j.1440-1746.2008.05395.x . [DOI] [PubMed] [Google Scholar]
- 47.Beneduce L, Pesce G, Gallotta A, Zampieri F, Biasiolo A, Tono N, et al. Tumour-specific induction of immune complexes: DCP-IgM in hepatocellular carcinoma. European journal of clinical investigation. 2008;38(8):571–7. Epub 2008/07/16. 10.1111/j.1365-2362.2008.01985.x . [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Long RE, Comunale MA, Junaidi O, Marrero J, Di Bisceglie AM, et al. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(6):1914–21. Epub 2009/05/21. 10.1158/1055-9965.epi-08-0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu JS, Wu DW, Liang S, Miao XY. GP73, a resident Golgi glycoprotein, is sensibility and specificity for hepatocellular carcinoma of diagnosis in a hepatitis B-endemic Asian population. Medical oncology (Northwood, London, England). 2010;27(2):339–45. Epub 2009/04/29. 10.1007/s12032-009-9215-y . [DOI] [PubMed] [Google Scholar]
- 50.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137(1):110–8. Epub 2009/04/14. 10.1053/j.gastro.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon YJ, Han KH, Kim DY. Role of serum prothrombin induced by vitamin K absence or antagonist-II in the early detection of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scandinavian journal of gastroenterology. 2009;44(7):861–6. Epub 2009/04/25. 10.1080/00365520902903034 . [DOI] [PubMed] [Google Scholar]
- 52.Sterling RK, Jeffers L, Gordon F, Venook AP, Reddy KR, Satomura S, et al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2009;7(1):104–13. Epub 2008/10/14. 10.1016/j.cgh.2008.08.041 . [DOI] [PubMed] [Google Scholar]
- 53.Baek YH, Lee JH, Jang JS, Lee SW, Han JY, Jeong JS, et al. Diagnostic role and correlation with staging systems of PIVKA-II compared with AFP. Hepato-gastroenterology. 2009;56(91–92):763–7. Epub 2009/07/23. . [PubMed] [Google Scholar]
- 54.Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. Journal of gastroenterology. 2010;45(12):1272–82. Epub 2010/07/14. 10.1007/s00535-010-0278-5 . [DOI] [PubMed] [Google Scholar]
- 55.Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59(12):1687–93. Epub 2010/09/30. 10.1136/gut.2010.214916 . [DOI] [PubMed] [Google Scholar]
- 56.Ozkan H, Erdal H, Tutkak H, Karaeren Z, Yakut M, Yuksel O, et al. Diagnostic and prognostic validity of Golgi protein 73 in hepatocellular carcinoma. Digestion. 2011;83(1–2):83–8. Epub 2010/11/03. 10.1159/000320379 . [DOI] [PubMed] [Google Scholar]
- 57.El-Din Bessa SS, Elwan NM, Suliman GA, El-Shourbagy SH. Clinical significance of plasma osteopontin level in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. Archives of medical research. 2010;41(7):541–7. Epub 2010/12/21. 10.1016/j.arcmed.2010.10.007 . [DOI] [PubMed] [Google Scholar]
- 58.Sharma B, Srinivasan R, Chawla YK, Kapil S, Saini N, Singla B, et al. Clinical utility of prothrombin induced by vitamin K absence in the detection of hepatocellular carcinoma in Indian population. Hepatology international. 2010;4(3):569–76. Epub 2010/11/11. 10.1007/s12072-010-9186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishida H, Matsuo S, Inoue Y. [Evaluation of diagnostic performance of alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) for HCV related hepatocellular carcinoma developed after long-term follow up]. Rinsho byori The Japanese journal of clinical pathology. 2010;58(11):1065–72. Epub 2011/01/15. . [PubMed] [Google Scholar]
- 60.Tian L, Wang Y, Xu D, Gui J, Jia X, Tong H, et al. Serological AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic diseases. International journal of cancer. 2011;129(8):1923–31. Epub 2010/12/09. 10.1002/ijc.25838 . [DOI] [PubMed] [Google Scholar]
- 61.Shi Y, Chen J, Li L, Sun Z, Zen L, Xu S, et al. A study of diagnostic value of golgi protein GP73 and its genetic assay in primary hepatic carcinoma. Technology in cancer research & treatment. 2011;10(3):287–94. Epub 2011/04/27. 10.7785/tcrt.2012.500205 . [DOI] [PubMed] [Google Scholar]
- 62.Abu El Makarem MA, Abdel-Aleem A, Ali A, Saber R, Shatat M, Rahem DA, et al. Diagnostic significance of plasma osteopontin in hepatitis C virus-related hepatocellular carcinoma. Annals of hepatology. 2011;10(3):296–305. Epub 2011/06/17. . [PubMed] [Google Scholar]
- 63.Morota K, Nakagawa M, Sekiya R, Hemken PM, Sokoll LJ, Elliott D, et al. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, alpha-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab Med. 2011;49(4):711–8. Epub 2011/01/15. 10.1515/CCLM.2011.097 . [DOI] [PubMed] [Google Scholar]
- 64.Salem M, Atti SA, Raziky ME, Darweesh SK, Sharkawy ME. Clinical Significance of Plasma Osteopontin Level as a Biomarker of Hepatocellular Carcinoma. Gastroenterology research. 2013;6(5):191–9. Epub 2013/10/01. 10.4021/gr499w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology (Baltimore, Md). 2012;55(2):483–90. Epub 2011/09/29. 10.1002/hep.24703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L, Rong W, Xiao T, Zhang Y, Xu B, Liu Y, et al. Secretory/releasing proteome-based identification of plasma biomarkers in HBV-associated hepatocellular carcinoma. Science China Life sciences. 2013;56(7):638–46. Epub 2013/06/12. 10.1007/s11427-013-4497-x . [DOI] [PubMed] [Google Scholar]
- 67.Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, et al. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World journal of gastroenterology. 2013;19(3):339–46. Epub 2013/02/02. 10.3748/wjg.v19.i3.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, et al. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87(2):121–31. Epub 2013/02/15. 10.1159/000346080 . [DOI] [PubMed] [Google Scholar]
- 69.Xu JB, Qi FZ, Xu G, Chen GF, Qin LX, Zhang JH. Value of alpha-fetoprotein and clinical characteristics in patients with liver neoplasm. Neoplasma. 2014;61(2):218–24. Epub 2013/12/05. 10.4149/neo_2014_028 . [DOI] [PubMed] [Google Scholar]
- 70.Chan SL, Mo F, Johnson PJ, Siu DY, Chan MH, Lau WY, et al. Performance of serum alpha-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2014;16(4):366–72. Epub 2013/08/29. 10.1111/hpb.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gopal P, Yopp AC, Waljee AK, Chiang J, Nehra M, Kandunoori P, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2014;12(5):870–7. Epub 2013/10/08. 10.1016/j.cgh.2013.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HJ, Yeon JE, Suh SJ, Lee SJ, Yoon EL, Kang K, et al. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut and liver. 2014;8(2):177–85. Epub 2014/03/29. 10.5009/gnl.2014.8.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nabih MI, Aref WM, Fathy MM. Significance of plasma osteopontin in diagnosis of hepatitis C virus-related hepatocellular carcinoma. Arab journal of gastroenterology: the official publication of the Pan-Arab Association of Gastroenterology. 2014;15(3–4):103–7. Epub 2014/09/25. 10.1016/j.ajg.2014.08.002 . [DOI] [PubMed] [Google Scholar]
- 74.Song P, Feng X, Inagaki Y, Song T, Zhang K, Wang Z, et al. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Bioscience trends. 2014;8(5):266–73. Epub 2014/11/11. 10.5582/bst.2014.01116 . [DOI] [PubMed] [Google Scholar]
- 75.da Costa AN, Plymoth A, Santos-Silva D, Ortiz-Cuaran S, Camey S, Guilloreau P, et al. Osteopontin and latent-TGF beta binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. International journal of cancer. 2015;136(1):172–81. Epub 2014/05/08. 10.1002/ijc.28953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pote N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62(4):848–54. Epub 2014/12/03. 10.1016/j.jhep.2014.11.005 . [DOI] [PubMed] [Google Scholar]
- 77.Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC, et al. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. The American journal of gastroenterology. 2015;110(6):836–44; quiz 45. Epub 2015/04/15. 10.1038/ajg.2015.100 . [DOI] [PubMed] [Google Scholar]
- 78.Gani RA, Suryamin M, Hasan I, Lesmana CR, Sanityoso A. Performance of Alpha Fetoprotein in Combination with Alpha-1-acid Glycoprotein for Diagnosis of Hepatocellular Carcinoma Among Liver Cirrhosis Patients. Acta medica Indonesiana. 2015;47(3):216–22. Epub 2015/11/21. . [PubMed] [Google Scholar]
- 79.Chimparlee N, Chuaypen N, Khlaiphuengsin A, Pinjaroen N, Payungporn S, Poovorawan Y, et al. Diagnostic and Prognostic Roles of Serum Osteopontin and Osteopontin Promoter Polymorphisms in Hepatitis B-related Hepatocellular Carcinoma. Asian Pacific journal of cancer prevention: APJCP. 2015;16(16):7211–7. Epub 2015/10/31. 10.7314/apjcp.2015.16.16.7211 . [DOI] [PubMed] [Google Scholar]
- 80.Fouad SA, Mohamed NA, Fawzy MW, Moustafa DA. Plasma Osteopontin Level in Chronic Liver Disease and Hepatocellular Carcinoma. Hepatitis monthly. 2015;15(9):e30753 Epub 2015/10/27. 10.5812/hepatmon.30753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ge T, Shen Q, Wang N, Zhang Y, Ge Z, Chu W, et al. Diagnostic values of alpha-fetoprotein, dickkopf-1, and osteopontin for hepatocellular carcinoma. Medical oncology (Northwood, London, England). 2015;32(3):59 Epub 2015/02/06. 10.1007/s12032-014-0367-z . [DOI] [PubMed] [Google Scholar]
- 82.Yu R, Ding S, Tan W, Tan S, Tan Z, Xiang S, et al. Performance of Protein Induced by Vitamin K Absence or Antagonist-II (PIVKA-II) for Hepatocellular Carcinoma Screening in Chinese Population. Hepatitis monthly. 2015;15(7):e28806 Epub 2015/08/25. 10.5812/hepatmon.28806v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jang ES, Jeong SH, Kim JW, Choi YS, Leissner P, Brechot C. Diagnostic Performance of Alpha-Fetoprotein, Protein Induced by Vitamin K Absence, Osteopontin, Dickkopf-1 and Its Combinations for Hepatocellular Carcinoma. PloS one. 2016;11(3):e0151069 Epub 2016/03/18. 10.1371/journal.pone.0151069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vongsuvanh R, van der Poorten D, Iseli T, Strasser SI, McCaughan GW, George J. Midkine Increases Diagnostic Yield in AFP Negative and NASH-Related Hepatocellular Carcinoma. PloS one. 2016;11(5):e0155800 Epub 2016/05/25. 10.1371/journal.pone.0155800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji J, Wang H, Li Y, Zheng L, Yin Y, Zou Z, et al. Diagnostic Evaluation of Des-Gamma-Carboxy Prothrombin versus alpha-Fetoprotein for Hepatitis B Virus-Related Hepatocellular Carcinoma in China: A Large-Scale, Multicentre Study. PloS one. 2016;11(4):e0153227 Epub 2016/04/14. 10.1371/journal.pone.0153227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn DG, Kim HJ, Kang H, Lee HW, Bae SH, Lee JH, et al. Feasibility of alpha-fetoprotein as a diagnostic tool for hepatocellular carcinoma in Korea. The Korean journal of internal medicine. 2016;31(1):46–53. Epub 2016/01/16. 10.3904/kjim.2016.31.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim TS, Kim DY, Han KH, Kim HS, Shin SH, Jung KS, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scandinavian journal of gastroenterology. 2016;51(3):344–53. Epub 2015/09/05. 10.3109/00365521.2015.1082190 . [DOI] [PubMed] [Google Scholar]
- 88.Petitti DB. Approaches to heterogeneity in meta-analysis. Statistics in medicine. 2001;20(23):3625–33. Epub 2001/12/18. 10.1002/sim.1091 . [DOI] [PubMed] [Google Scholar]
- 89.Liu YR, Lin BB, Zeng DW, Zhu YY, Chen J, Zheng Q, et al. Alpha-fetoprotein level as a biomarker of liver fibrosis status: a cross-sectional study of 619 consecutive patients with chronic hepatitis B. BMC gastroenterology. 2014;14:145 Epub 2014/08/17. 10.1186/1471-230X-14-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Xiaoman, Wei Meijuan, Xu Zhengju, et al. , Expression characteristics of serum alpha-fetoprotein, interleukin-6 and golgi body protein 73 in liver diseases and their diagnostic value in hepatocellular carcinoma. Chinese journal of experimental and clinical infectious diseases (electronic version), 2017. 11(04): p. 339–344. (in Chinese) [Google Scholar]
- 91.Reim D, Choi YS, Yoon HM, Park B, Eom BW, Kook MC, et al. Alpha-fetoprotein is a significant prognostic factor for gastric cancer: Results from a propensity score matching analysis after curative resection. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2017;43(8):1542–9. Epub 2017/05/18. 10.1016/j.ejso.2017.04.005 . [DOI] [PubMed] [Google Scholar]
- 92.Lu Ningning, Zhang Yinghua, Wang Haiyan, et al. , Serum AFP vitamin K deficiency or antagonist Ⅱ induced proteins in the diagnosis of hepatocellular carcinoma (HCC) pathological application value. Journal of clinical hepatobiliary disease, 2017. 33(11): p. 2162–2165. (in Chinese) [Google Scholar]
- 93.Li Wei, Bai Yun, Li Yunting, et al. , Clinical application and laboratory evaluation of alpha-fetoprotein. Clinical misdiagnosis and mistreatment, 2007(08): p. 97–100. (in Chinese) [Google Scholar]
- 94.Li Zhao, Zhu Jiye. Interpretation of primary liver cancer diagnosis and treatment norms (2017 edition). Journal of clinical hepatobiliary disease, 2017. 33(09): p. 1655–1657. (in Chinese) [Google Scholar]
- 95.Cedrone A, Covino M, Caturelli E, Pompili M, Lorenzelli G, Villani MR, et al. Utility of alpha-fetoprotein (AFP) in the screening of patients with virus-related chronic liver disease: does different viral etiology influence AFP levels in HCC? A study in 350 western patients. Hepato-gastroenterology. 2000;47(36):1654–8. Epub 2001/01/10. . [PubMed] [Google Scholar]
- 96.Xu Jianye, Yi Ling, Ran Chongxin, et al. , Study on serum alpha fetoprotein in diagnosis of primary liver cancer. Chongqing medical, 2009. 38(01): p. 51–52+55. (in Chinese) [Google Scholar]
- 97.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md). 2011;53(3):1020–2. Epub 2011/03/05. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Qingru, Ji Shundong. The application value and clinical significance of tumor marker protein chip detection in the health examination of the elderly. Journal of jilin university (medical edition), 2016. 42(03): p. 535–540. (in Chinese) [Google Scholar]
- 99.Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC, et al. Corrigendum: Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis. The American journal of gastroenterology. 2016;111(11):1668 Epub 2016/11/04. 10.1038/ajg.2016.426 . [DOI] [PubMed] [Google Scholar]
- 100.Lai Bingyan, Li Fen. Comparison of CT and B ultrasound in the first diagnosis of primary liver cancer. Chinese journal of CT and MRI, 2016. 14(04): p. 71–73. (in Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.