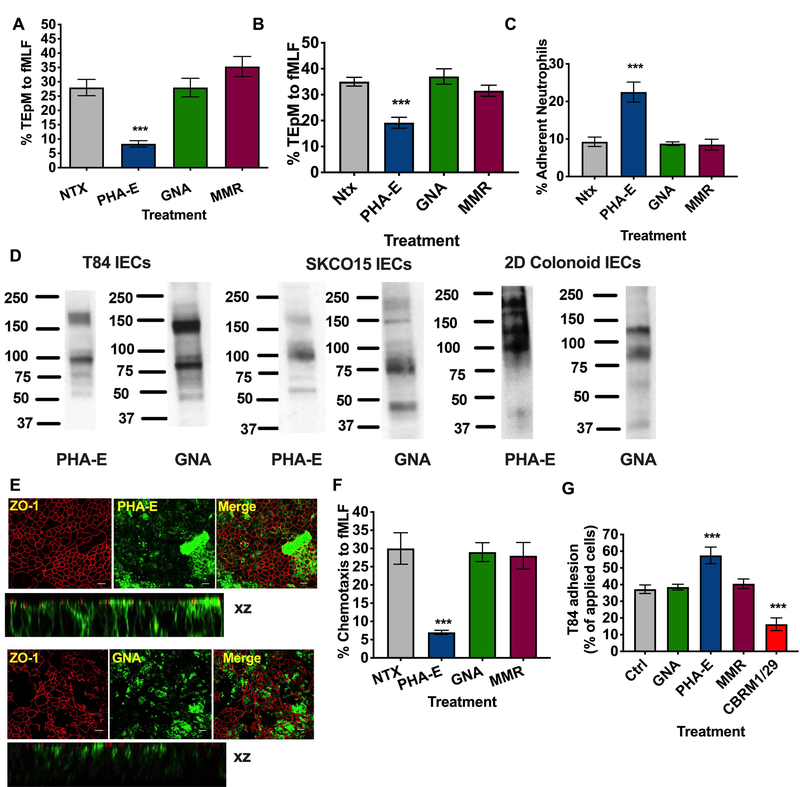
Figure 3. Targeting of CD11b glycans blocks PMN chemotaxis and TEpM and increases PMN adhesive interactions.
(A) T84 Intestinal epithelial cells were cultured to confluency on porous polycarbonate filters (Transwell). Human PMNs were applied to the upper chamber of transwell filters, incubated with 10μg/ml PHA-E or 10μg/ml GNA or 10μg/ml MMR and induced to migrate into the bottom chamber in response to 100nm fMLF. Migration was quantified by MPO assay (n=6 independent donors. ***, p<0.001). Effect of addition of 10μg/ml PHA-E, 10μg/ml GNA or 10μg/ml MMR to the apical epithelial surface on PMN TEpM (B) and PMN adherence to the apical epithelial surface (C). (D) Expression of glycans recognized by PHA-E and GNA by immunoblotting of whole cell lysates from T84 IECs, SKCO15 IECs and differentiated human colonoids. (E) Fixed and permeabilized T84 IECs were stained with 10μg/ml anti- ZO-1 mAb (in red) and or 10μg/ml PHA-E/GNA (in green) and analyzed by confocal microscopy. Original magnification x 60, scale bar = 20μm. Representative images from n=3 experiments are shown en face or in the xz plane of section. (F) PMN chemotaxis was quantified by MPO following incubation of human PMN with 10μg/ml PHA-E, 10μg/ml GNA or 10μg/ml MMR. Data depict means ± SEM (A-C, G). n=4 independent donors ***, p<0.001. (G) CD11b/CD18 was added to Nunc maxisorp plates at a concentration of 5μg/ml before addition of 2.5 × 105 BCECF labeled T84 IECs treated with 10μg/ml indicated lectins (PHA-E, GNA, MMR) or blocking CD11b mAb (CBRM1/29). Following washing CD11b/CD18-IEC adhesion was quantified by measuring fluorescence at 485nm. Data shown are % adhesion of applied cells and depict means ± SEM (n=4) ***, p<0.001.