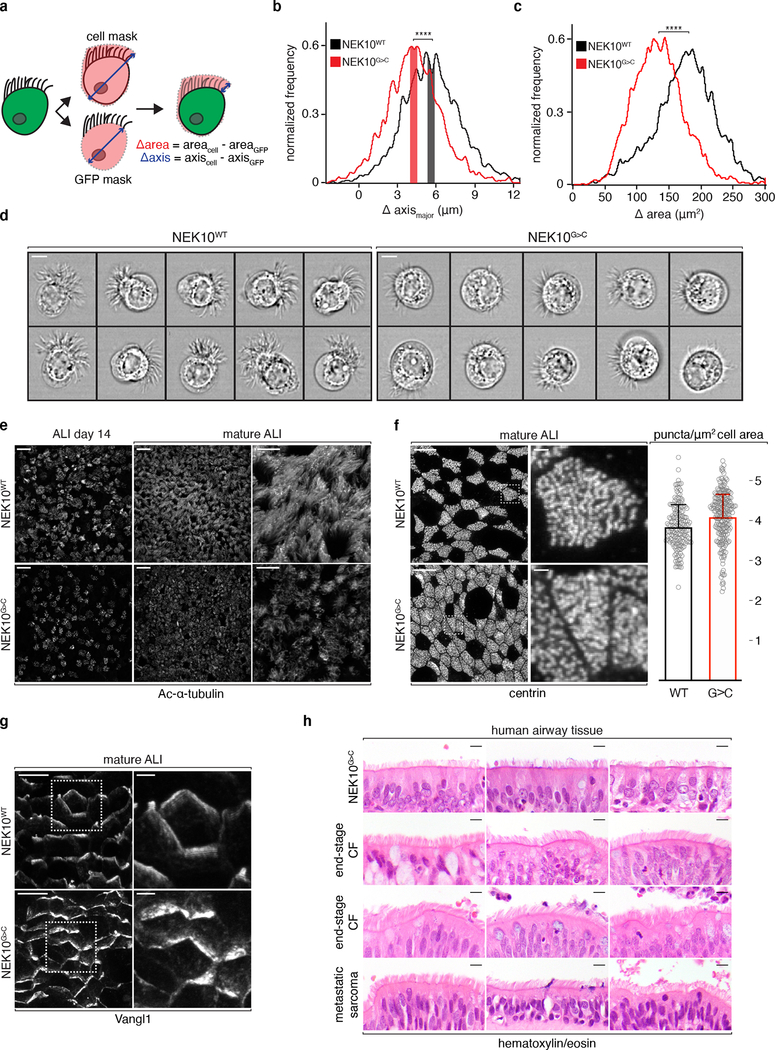
Fig. 3: Morphologically abnormal ciliated cells in NEK10-deficient airway.
a, schematic masking workflow for IFC morphological analysis. b, histogram of ciliary zone thickness of mature ALI MCCs of the indicated genotypes, n=4108 (NEK10WT), 3513 (NEK10G>C), shaded bars indicate medians ±0.25μm. c, histogram of ciliary area of mature ALI MCCs of the indicated genotypes, n=4108 (NEK10WT), 3513 (NEK10G>C). d, single cell images taken from the shaded regions in (b), scale bars 7μm. e, confocal maximum intensity projections (MIPs) of ALI of the indicated genotype and maturity following IF against Ac-α-tubulin, representative of 3 independent ALI differentiations, scale bars 25μm (left 4 panels) and 10μm (right 2 panels). f, confocal MIPs of mature ALI after IF against basal body marker centrin, dashed boxes mark full resolution regions in middle panels, scale bars 10μm (left 2 panels) and 1μm (middle 2 panels); column graph: centrin puncta per μm2 (mean ±S.D.) of ciliated cell surface area, n=71 cells and 10,855 puncta (NEK10WT), 38 cells and 5,369 puncta (NEK10G>C) pooled from 4 independent ALI differentiations. g, confocal MIPs of mature ALI after IF against PCP marker Vangl1, dashed boxes mark full resolution regions in right panel, scale bars 10μm (left panels) and 2.5μm (right panels), representative of 3 independent ALI differentiations. h, hematoxylin/eosin stained human large airway tissue; upper 3 samples taken from lung explants during transplantation for end-stage bronchiectasis due to the indicated etiologies, 4th sample from patient undergoing resection for an unrelated diagnosis, scale bars 5μm. ****p≤0.0001.