FIGURE 4.
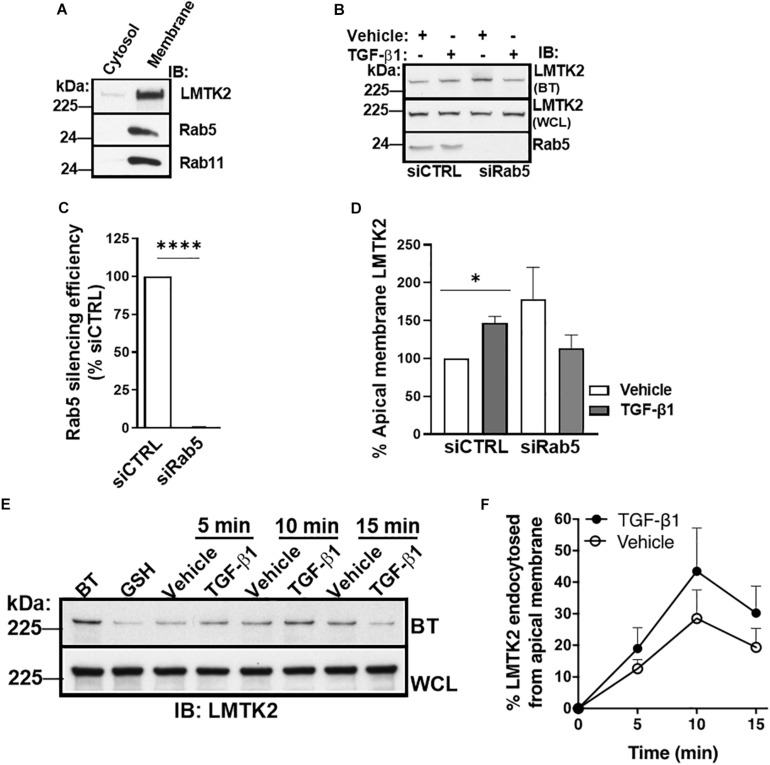
TGF-β1 did not inhibit LMTK2 endocytosis. Representative immunoblots of LMTK2, Rab11 and Rab5 abundance in both cytosol and membrane fractions (A). CFBE41o- cells were cultured on Transwell filters to allow polarization. Subcellular fractionation was performed through ultracentrifugation (100,000 x g) and using discontinuous sucrose gradient (28%/50% sucrose interface). Cytosol and membrane fractions of the cells were isolated and protein levels were measured. LMTK2, Rab5 and Rab11 were enriched at the membrane fraction of the cells. Representative immunoblots (B) and summary of experiments (C,D) demonstrating that Rab5-dependent endocytosis is not affected by TGF-β1 stimulation. CFBE41o- cells were cultured on Transwell filters to allow polarization, transfected with Rab5-specific siRNA (siRab5) or non-targeting control siRNA (siCTRL) and treated with TGF-β1 (15 ng/ml) or its vehicle for 1 h. Apical membrane proteins were isolated by cell surface biotinylation. We observed an increase of LMTK2 abundance at the apical membrane after TGF-β1 in siNeg-transfected CFBE41o- cells that is disrupted after impairment of Rab5-dependent endocytosis. Apical LMTK2 levels were normalized to total LMTK2 levels present in the whole cell lysate. Three experiments/group. Representative immunoblots (E) and summary of experiments (F) showing the effects of TGF-β1 on CFTR endocytosis. Apical membrane proteins of polarized CFBE41o- cells were biotinylated, and endocytosis was induced at 37°C with TGF-β1 or vehicle treatment for 5, 10, and 15 min. LMTK2 endocytosis peaked at 10 min. TGF-β1 tended to increase the endocytosis. Internalized LMTK2 was normalized for total LMTK2 abundance. Five experiments/group. ∗p < 0.05; ∗∗∗∗p < 0.0001. Error bars, SEM. BT, biotinylation; IB, immunoblot; WCL, whole cell lysate.