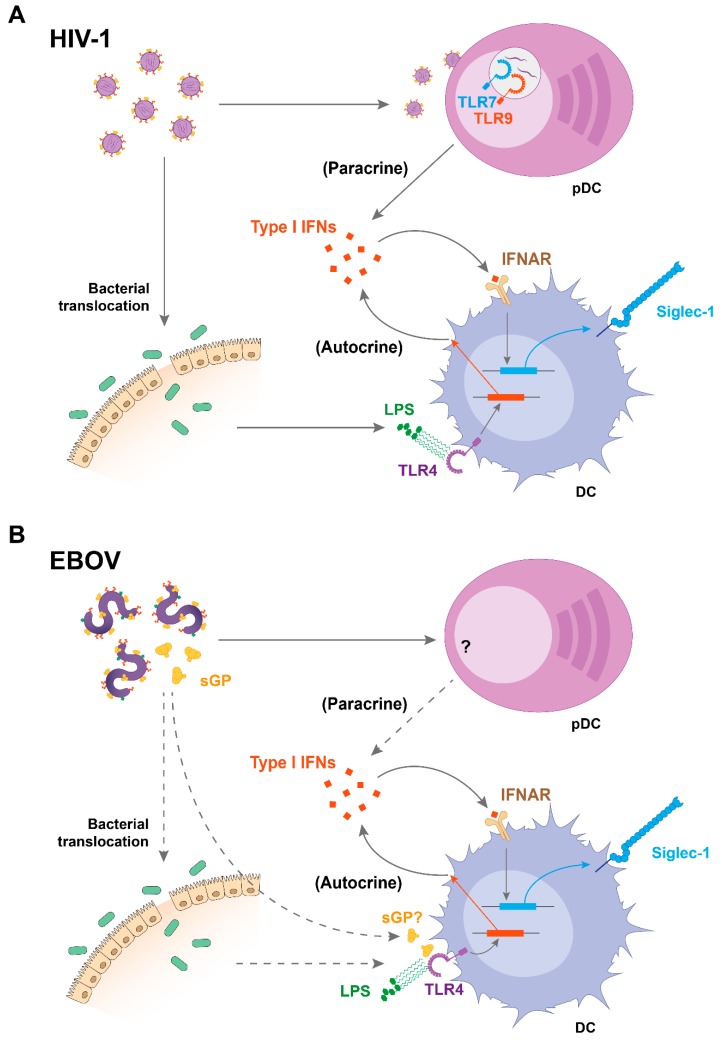
Figure 1.
Mechanisms of Siglec-1 upregulation during human immunodeficiency virus (HIV)-1 and Ebola virus (EBOV) infections. (A) HIV-1 induces secretion of type I interferons (IFNs) by plasmacytoid DCs (pDCs) through Toll-like receptor (TLR) -7 and -9 sensing, which upregulates Siglec-1 on DCs in a paracrine manner. In addition, lipopolysaccharide (LPS) from bacterial translocation upregulates Siglec-1 on DCs via TLR4 sensing and autocrine type I IFN release. (B) During EBOV infection, type I IFNs might also play a central role in enhancing Siglec-1 expression on DCs, although this needs further investigation. pDCs may produce type I IFNs in response to EBOV infection in vivo, while bacterial translocation was suspected during a case of gram-negative septicemia in an EBOV-infected patient. In parallel, viral components such as secreted EBOV glycoprotein may induce activation of myeloid cells through TLR4 signaling, providing an alternative stimulus of autocrine type I IFNs during EBOV infection. While solid arrows indicate established mechanisms, dotted arrows suggest processes that require further investigation. IFNAR: IFNα/β receptor; sGP: secreted glycoprotein.