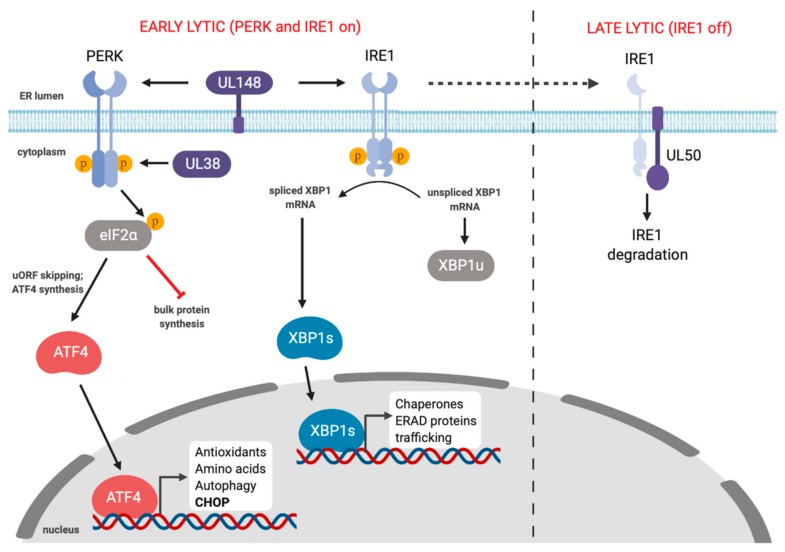
Figure 4.
Differential control of the unfolded protein response by HCMV. In the early stages of HCMV lytic replication, PERK is activated by UL148 and UL38, which causes eIF2α phosphorylation and diminished global protein synthesis. At the same time, stress-dependent uORF skipping enables translation of the ATF4 bZIP transcription factor, which translocates to the nucleus and transactivates a variety of UPR genes. UL148 activates PERK as well as IRE1, which enables XBP1 splicing and synthesis of the XBP1s bZIP transcription factor that transactivates a distinct set of UPR genes. Later in the lytic cycle, the UL50 protein binds and downregulates IRE1 through an unknown mechanism, effectively shutting down IRE1-dependent UPR gene expression.