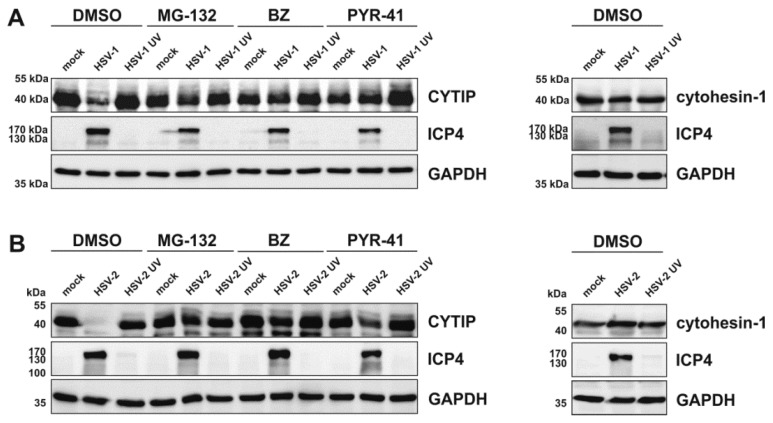
Figure 11.
HSV-1 and HSV-2 induce proteasome- and ubiquitin-dependent degradation of CYTIP in transfected HEK293T cells. HEK293T cells were transfected with 1 µg of plasmid DNA encoding CYTIP (left panels) or cytohesin-1 (right panels). After 24 h, cells were mock-treated or infected with (A) HSV-1 (MOI of 2) or HSV-1 UV-inactivated virions (MOI of 2; 8 × 0.12 J/cm2) and (B) HSV-2 (MOI of 5) or HSV-2 UV-inactivated virions (MOI of 5; 8 × 0.12 J/cm2). Cells were treated with or without the proteasomal inhibitors MG-132 (10 µM) or bortezomib (BZ; 2 µM) 1 hpi, or the ubiquitination inhibitor PYR-41 (80 µM) 4 hpi. As control, cells were treated with DMSO. Cells were harvested 18 hpi and protein lysates were subjected to Western blot analysis using equal amounts of protein loaded on a 12% acrylamide SDS-gel. Expression levels of CYTIP, cytohesin-1, ICP4 as infection control, or GAPDH as loading control, were monitored using specific antibodies. The experiment was performed three times independently and representative data are shown.