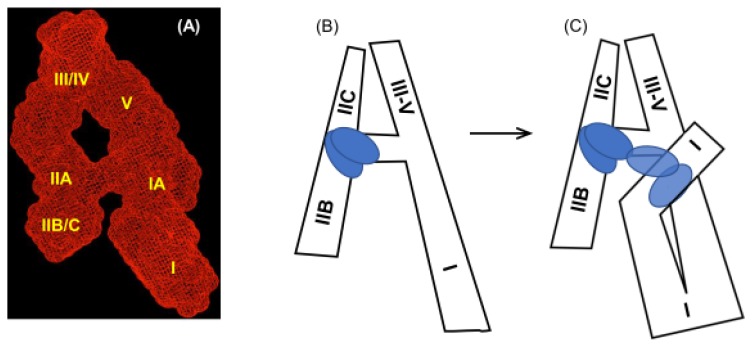
Figure 5.
Conformational flexibility of HIV-1 RRE SL-I contributes towards assembly of the Rev-mediated export complex. (A) Molecular envelope of the RRE RNA, drawn in mesh and derived by SAXS [32]. The spatial resolution of the envelope is 21 A°. (B) Cartoon representation of the RRE, depicting assembly initiating via a single nucleation point in SL-II for two Rev molecules (blue). (C) Through an SL-I conformational change, “coupling” of SL-I and SL-II Rev-binding sites promote a tetrameric intermediate complex proposed to serve as a specificity checkpoint. Rev and the RRE could thereafter simultaneously sample a number of interaction conformations until an optimal binding state for Crm1 binding and nuclear export is attained.