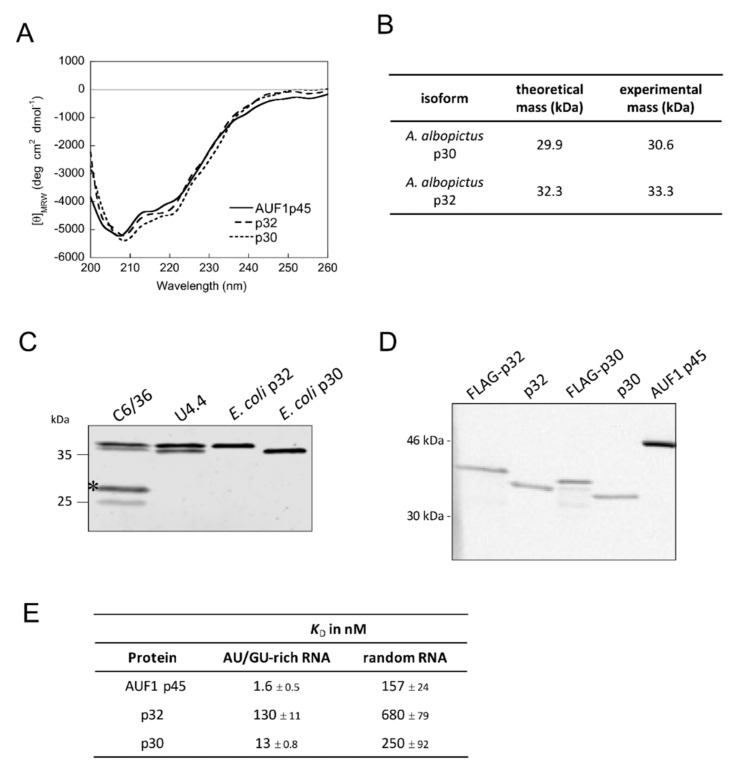
Figure 6.
Characterization of mosquito proteins p30 and p32. (A) Far-UV circular dichroism (CD) spectra of AUF1 p45 and mosquito p30 and p32 were recorded. The acquired data were normalized to mean residue weight (MRW) ellipticities. The CD data for AUF1 p45 were taken from [16]. (B) Summary of the analytical ultracentrifugation experiments demonstrating that mosquito proteins p30 and p32 are monomeric proteins (see Supplementary Figure S3). (C) Detection of squid isoforms p30 and p32 in cell extracts of mosquito cell lines C6/36 and U4.4. An antibody that was raised against the full-length p32 protein (purified from E. coli) was applied. The asterisk indicates bands that most likely correspond to degradation products of p30. (D) In vitro methylation assay with PRMT1 and different protein preparations. Equal amounts (1 pmol) of mosquito proteins p30 and p32 that were purified from E. coli, as well as FLAG-p30 and FLAG-p32 proteins that were purified from C6/36 cells, were methylated by PRMT1. AUF1 p45 (13 pmol) that was purified from E. coli served as a positive control. The samples were taken after 2 h and analyzed by SDS–PAGE and phosphor imaging. (E) RNA binding affinities of human AUF1 p45 and mosquito proteins p30 and p32 to an AU/GU-rich RNA and a randomly composed RNA. Dissociation constants and standard deviations derived from at least three measurements. The binding data for AUF1 p45 were taken from [16].