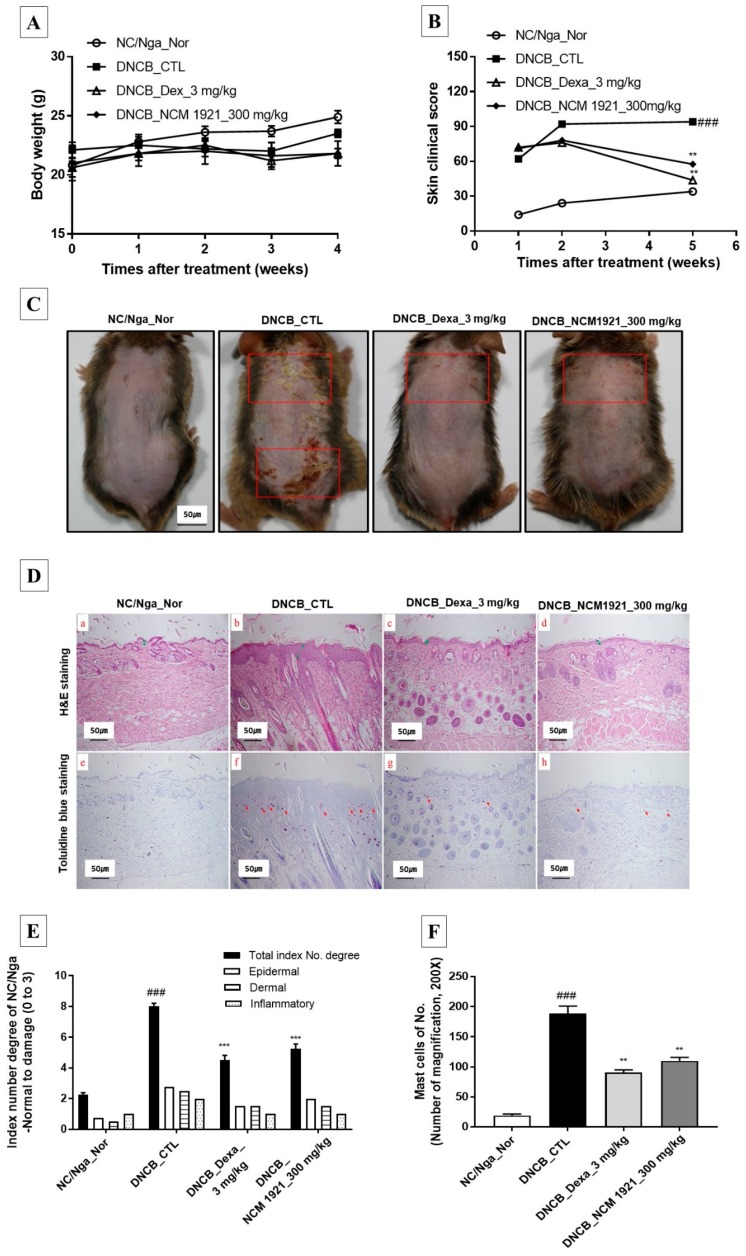
Figure 1.
The effects of NCM 1921 on the development and the histological manifestations of atopic dermatitis in NC/Nga mice. (A) Body weight was measured once a week. (B) The severity of clinical symptoms of atopic dermatitis was evaluated macroscopically and calculated as the sum of the individual scores for the following four atopic dermatitis signs and symptoms: erythema/hemorrhage, edema, excoriation/erosion, and scaling/dryness. (C) Macroscopic lesions (square) in NC/Nga with atopic dermatitis induced via topical application of DNCB. (D) Dorsal skin sections were stained with H&E (a–d) and TB (e–h). (E) Total index number of degree, and (F) mast cell number in the dorsal skin were quantitated. H&E, hematoxylin-eosin stain; TB, toluidine blue stain; NC/Nga_Nr: normal control; DNCB_CTL: 1-chloro-2,4-dinitrobenzene (DNCB)-treated control; DNCB_Dexa 3 mg/kg: 3 mg/kg dexamethasone; DNCB_NCM 1921 300 mg/kg: 300 mg/kg NCM 1921. Values are expressed as the means ± SEMs (n = 6). ### p < 0.001 compared with NC/Nga_Nor; ** p < 0.01 and *** p < 0.001 compared with DNCB_CTL as determined by ANOVA followed by multiple comparison tests.